Conventional measures of obstructive sleep apnea (OSA) severity, such as the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) are commonly used to quantify OSA severity and guide therapeutical decision-making processes. However, it is widely recognized that both AHI and ODI have important limitations and novel physiologically-informed metrics are needed to better capture the severity of OSA and characterize its physiological consequences, particularly the severity of recurrent nocturnal hypoxemia, ensuing the respiratory events. According to recent studies, the sleep apnea-specific “hypoxic burden (HB)”, defined as the sum of individual areas under the oxygen desaturation curve, has shown some promise in identifying high risk individuals with OSA. In addition to the frequency of respiratory events, HB capture the depth and duration of OSA-related hypoxemia that may prove to be important disease characterizing features, not captured by the conventional “frequency-based” metrics, such as AHI and ODI. In this “perspective” paper the methods to quantify the HB, its characteristics, associations with health outcomes, and its limitations will be discussed.
The latest epidemiological studies estimate that one billion people suffer from obstructive sleep apnea (OSA), and half are of at least moderate severity.1 Studies in the general population2 indicate a substantial heterogeneity in OSA characteristics and symptoms, potentially due to factors which include age (the severity of sleep-disordered breathing [SDB] is greater in the elderly]),3–5 obesity (the higher the body mass index, the more severe the SDB),6 and sex/race among others.7 However, how OSA is defined and quantified are probably the most important variables contributing to the heterogeneity of OSA in general population.8,9 OSA is characterized by frequent partial (“hypopnea”) or complete (“apnea”) pauses in breathing during sleep. The severity of OSA is historically quantified by the apnea-hypopnea index (AHI) which is the total number of apneas and hypopneas per hour of sleep. The AHI is the most commonly used metric to diagnose OSA (by convention, an AHI>5events/h defines the disease) and quantify its severity.10 Based on this definition, 83.8% of men and 60.8% of women aged 35–75 years have OSA, which is probably an overestimation.2 Additionally, the metric AHI is not strongly correlated with symptoms,11 nocturnal oxygen desaturations (one of the key culprits linking OSA to multiple health outcomes), sleep fragmentation,12 or measures of quality of life.13
In addition to the AHI, other conventional measures of OSA severity, such as the “oxygen desaturation index (ODI)” are commonly used to characterize intermittent hypoxemia (IH), an OSA-related physiological consequence that is likely responsible for most of the pathophysiological systemic complications of OSA.14 While ODI and other conventional measures of hypoxemia commonly demonstrate a better relationship with adverse health outcomes than the AHI, they share many of the same limitations with AHI.14,15
A full-night polygraphic or polysomnographic study consists of an 8-h continuous recording of respiration, oxygen saturation, heart rate, body movements, etc. Yet, these signals are almost always reduced to simple frequency counts (e.g. AHI, ODI, arousal index) that disregard the depth and the duration of the respiratory events. This has led to recent efforts to develop and validate more quantitative metrics that can be automatically calculated and implemented in the clinical practice. The OSA-specific hypoxic burden (HB) that quantifies the frequency, depth, and duration of respiratory event-related desaturation has demonstrated significant associations with several adverse health outcomes in recent studies.16 In this article, we will provide a detailed review of hypoxic burden, its definition, properties, measurement, strengths, limitations and potential implementations for clinical practice. In addition, we summarize the association of HB with adverse health outcomes in OSA patients (particularly in the cardiovascular sphere). Finally, we will discuss future directions and potential ways to improve OSA characterization and quantification.
Limitations of the current measures of OSA definition, severity and prognosisInternational guidelines use the AHI as the standard metric to establish the diagnosis and determine the severity of OSA. This measure has also been used, to guide treatment decisions.10,17–19 AHI has also been the most commonly used metric to assess the impact of OSA on cardiovascular,20–27 neurocognitive,28 and metabolic29 outcomes and to assess the effect of treatment on these health consequences OSA is commonly diagnosed if the AHI≥5events/h and is categorized into mild, moderate, or severe if the AHI is 5–15, 15–30, or ≥30events/h, respectively.30,31 As stated above, alternative measures of OSA severity, including ODIs, have been also commonly used in the research and clinical settings to quantify the severity of hypoxemia.32–35
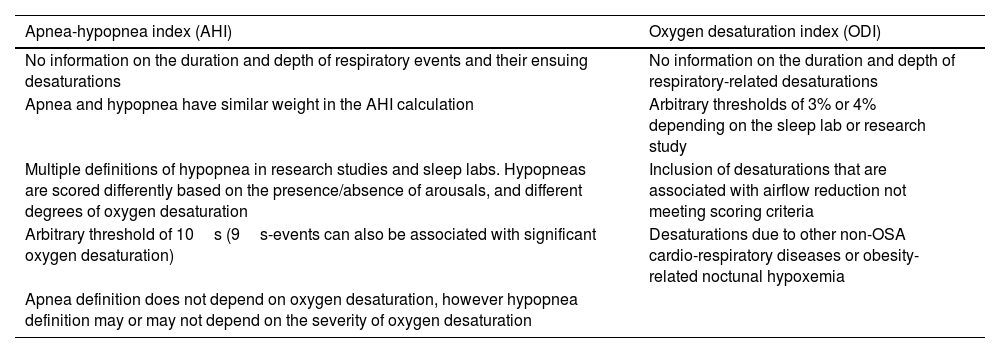
However, it is widely recognized that both AHI and ODI have important limitations (Table 1).36–38
Weaknesses of the apnea-hypopnea index and the oxygen desaturation index in establishing the diagnosis and quantifying the severity of obstructive sleep apnea (OSA).
| Apnea-hypopnea index (AHI) | Oxygen desaturation index (ODI) |
|---|---|
| No information on the duration and depth of respiratory events and their ensuing desaturations | No information on the duration and depth of respiratory-related desaturations |
| Apnea and hypopnea have similar weight in the AHI calculation | Arbitrary thresholds of 3% or 4% depending on the sleep lab or research study |
| Multiple definitions of hypopnea in research studies and sleep labs. Hypopneas are scored differently based on the presence/absence of arousals, and different degrees of oxygen desaturation | Inclusion of desaturations that are associated with airflow reduction not meeting scoring criteria |
| Arbitrary threshold of 10s (9s-events can also be associated with significant oxygen desaturation) | Desaturations due to other non-OSA cardio-respiratory diseases or obesity-related noctunal hypoxemia |
| Apnea definition does not depend on oxygen desaturation, however hypopnea definition may or may not depend on the severity of oxygen desaturation |
These limitations could potentially explain the generally low relationship between frequency-based metrics such as AHI and ODI and some clinical aspects of the disease including quality of life measurements and hypersomnia.39–41 Nonetheless, in some studies, compared with AHI, measures of IH, including ODI, have shown superior predictive ability related to important outcomes,32–35 which highlights the need for better quantification of nocturnal hypoxemia using indices that captures all dimensions of OSA-related desaturations, including frequency, duration, and depth.
Duration and depth of OSA-specific oxygen desaturations. why are they important?Respiratory events are identified based on thresholds for duration (i.e. ≥10s) and depth (≥30% reduction from pre-event baseline airflow). However, beyond these thresholds the true characteristics of respiratory events are not captured in the AHI and ODI. These features of respiratory events are likely important not only in decisions regarding OSA management and risk stratification but also as key contributors to OSA-related symptoms.40
To delineate the effect and usefulness of measuring specific features of apneic episodes, past studies have suggested that incorporating the duration of respiratory events and their associated desaturation areas into the AHI could improve risk stratification in individuals with OSA.42 For example, studies have shown that longer37,43 and deeper44 apneas and hypopneas elicited a larger cardiovascular response than shorter and milder events. In addition, detailed analysis of the oxygen desaturations and their morphology predicted the progression of mild to more severe OSA. Indeed, longer and deeper desaturations were associated with the worsening of the disease45 over a 5-year period. All these studies provide evidence that incorporating the duration and depth of respiratory events and their associated desaturations could provide useful information in identifying and managing OSA patients with greater precision (precision medicine). Given that oxygen desaturation is readily available from in-lab and in-home sleep studies, incorporating the depth and duration of IH in a metric that has predictive value is of great interest. The OSA-specific HB16 is a candidate metric that was designed to capture the frequency of respiratory events and the depth and duration of their associated desaturation.
Definition of hypoxic burdenHB was defined as the total area under the oxygen saturation curve from a pre-event baseline oxygen desaturation.16 The calculation of HB for each individual is described below:
- 1.
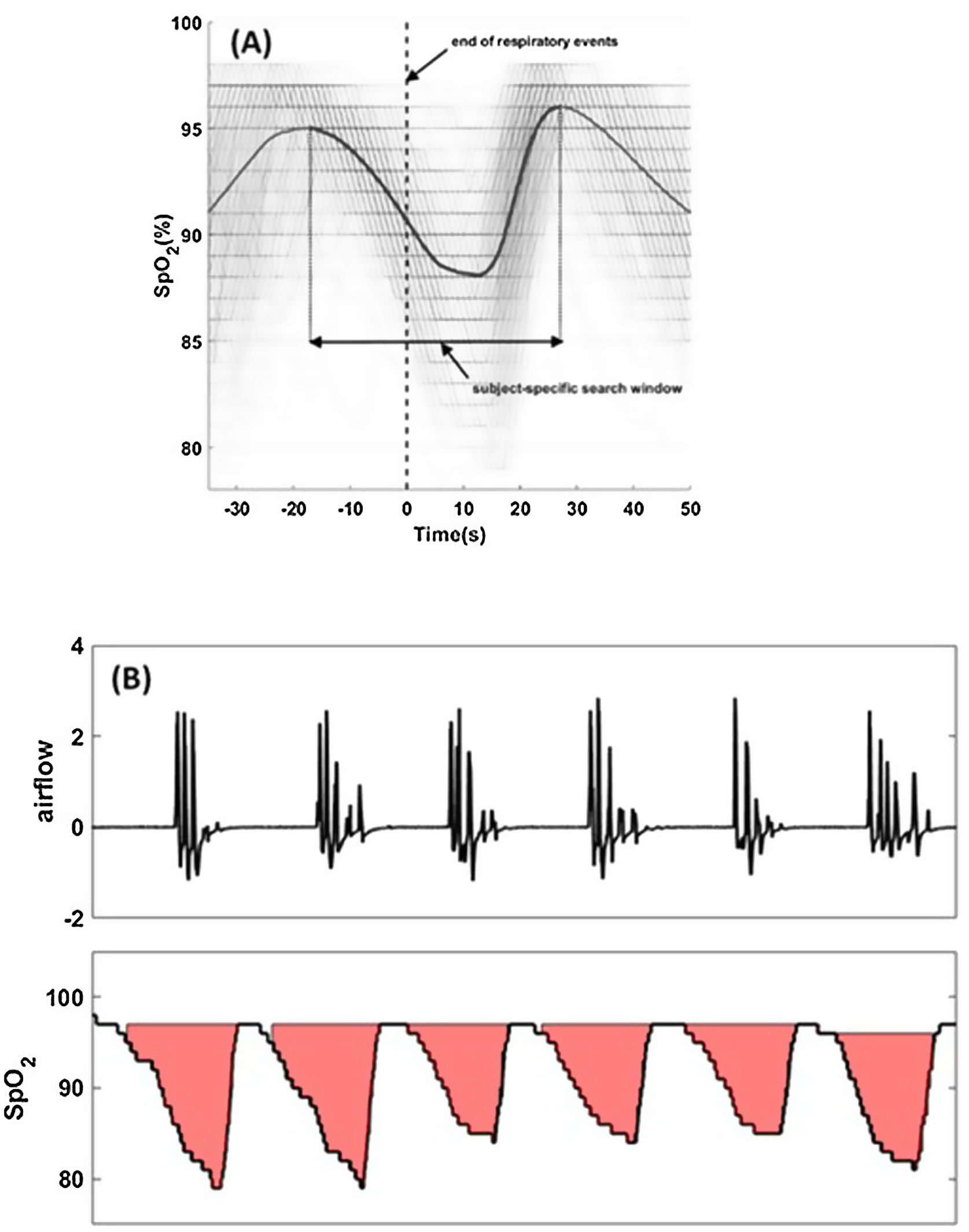
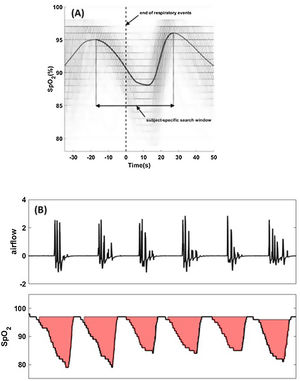
For each apnea and hypopnea (scored based on a 30% reduction in airflow), the termination of the event is called the synchronization time or “time-zero”. The oxygen saturation signals around time-zero (for all apneas and hypopneas) are synchronized with respect to time-zero (Fig. 1A).
Fig. 1.Hypoxic burden is determined by creating a subject-specific search window (panel (A)) from all respiratory events regardless of desaturation or arousals. Overlaid oxygen saturation signals (SpO2) associated with all respiratory events are synchronized at the termination of events (time zero). Synchronized SpO2 signals are averaged to quantify the subject-specific desaturation curve (solid black line). The search window is the time between the two peaks. The search window will be used to determine the area under individual desaturation curves (panel (B)). Total hypoxic burden (HB) is the sum of all individuals areas normalized by sleep time.
(0.17MB). - 2.
The time-aligned oxygen saturation signals are ensemble-averaged such that the mean value at each time point is calculated, resulting in a subject-specific average oxygen saturation curve specific to apneas and hypopneas (Fig. 1A).
- 3.
A subject-specific search window to quantify the area under the desaturation curve for each event is defined as the interval between the pre-event and post-event maximum oxygen saturation values (Fig. 1A). This time-locked search window will be used to determine the start and end of oxygen desaturation and calculate the area under desaturation curve for each respiratory event.
- 4.
Total HB (%min/h) is defined as the sum of individual areas (%min; Fig. 1B) divided by total sleep time (h).
Currently, there are two commercial softwares that include the HB calculation: Sleepware G· from Respironics, Inc, USA and Cidelec, Angers, France). There are no comparison studies between these software and the error measurement has not been still assessed due to the lack of a gold standard.
Other oxygen desaturation metrics used in OSAOther parameters such as ODI or percent sleep time with SpO2 below 90% (T90) are routinely measured from sleep studies. However, similar to AHI, ODI only measures the event rate and not the desaturation duration or depth. T90, on the other hand, only measures the desaturations that dip below an arbitrary threshold of 90%. In some individuals, for example, the desaturation starts from a high pre-event value such as 96% and never dip below 90%, yielding a T90 of zero. On the other hand, if baseline SpO2 is slightly below 90%, the T90 will be 100% regardless of the number and severity of desaturations. Desaturation severity parameter,46,47 proposed by Kulkas et al. was designed to determine the areas under the desaturation curve similar to the HB. However, this metric was based on the presence of at least a 4% desaturation. The desaturation severity metric does not take into account the 3% desaturations (common in milder form of OSA). In addition, it may overestimate the area when there is incomplete recovery of oxygen desaturation to pre-event baseline and because there is no linkage between scored desaturations and respiratory events, the desaturation severity parameter could potentially capture hypoxemia due to other non-OSA related cardiovascular or respiratory comorbidities.
Hypoxic burden and cardiovascular riskOSA is associated with a variety of cardiovascular diseases in large observational studies.20 In a seminal observational study by Marin et al.48 men with severe untreated OSA were shown to be at an increased risk of incident fatal and non-fatal cardiovascular events compared to healthy men while those who were treated with CPAP had a similar risk profile comparted to healthy men. However, other studies have been unable to show consistent associations between OSA and cardiovascular outcomes or CPAP benefit in secondary prevention studies of cardiovascular disease.49 As stated in the previous sections, one of the primary reasons for these inconsistencies may be related to the disease characterization using AHI. Measures of IH severity (both conventional and novel), on the other hand, tend to provide stronger associations with cardiovascular diseases, particularly hypertension and atherosclerosis.32–35
The association of OSA-specific HB with several cardiovascular outcomes have been assessed in past studies, summarized in Table 2. We excluded the study by Huang et al. since the HB developed by these authors, despite having the same unit, is almost 10 times lower for a given AHI and/or T90. Therefore, the methods are not comparable.50
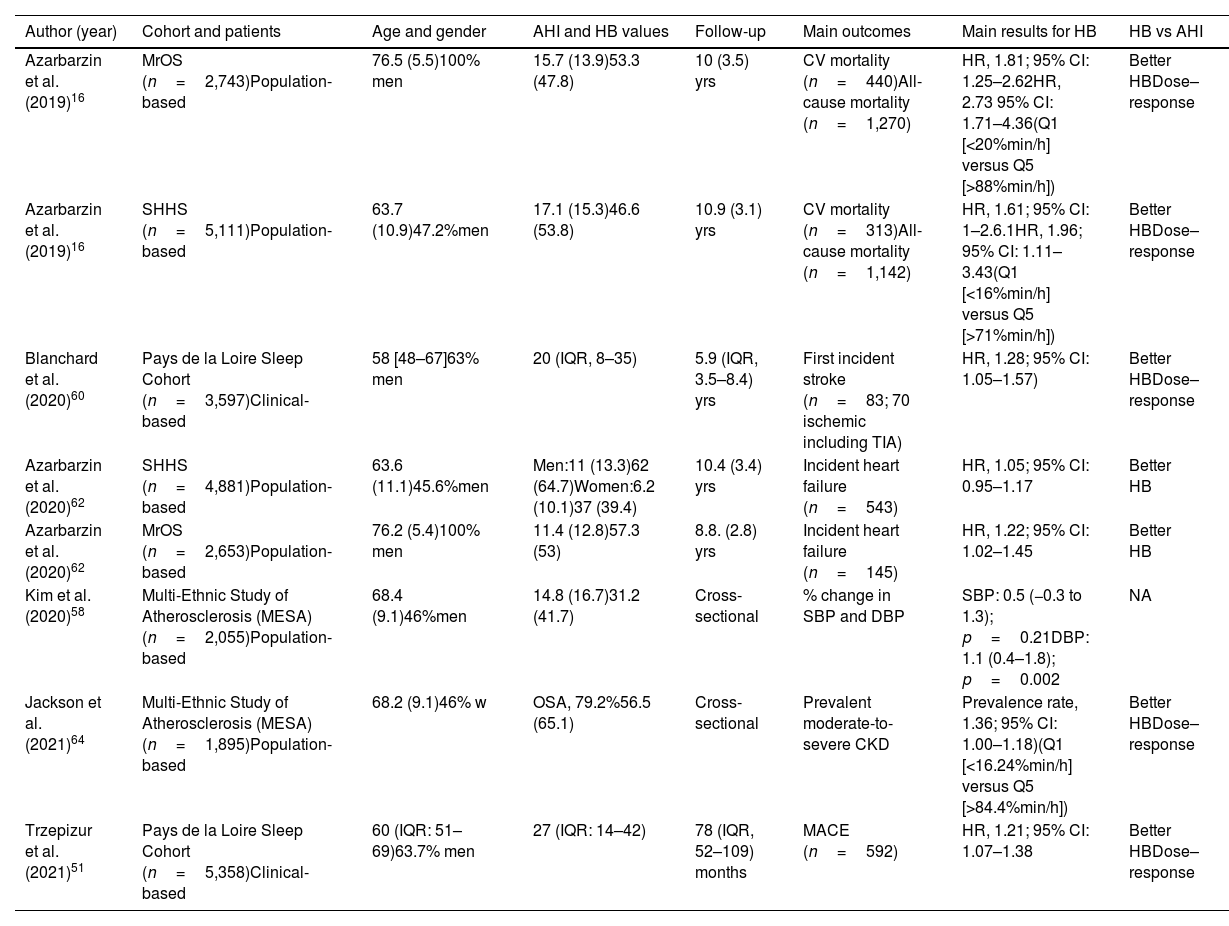
Main characteristics and results of studies including hypoxic burden as a measure of OSA severity.
| Author (year) | Cohort and patients | Age and gender | AHI and HB values | Follow-up | Main outcomes | Main results for HB | HB vs AHI |
|---|---|---|---|---|---|---|---|
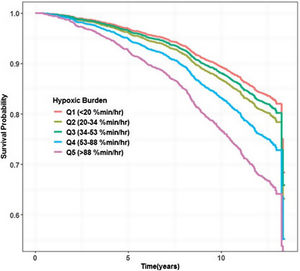
| Azarbarzin et al. (2019)16 | MrOS (n=2,743)Population-based | 76.5 (5.5)100% men | 15.7 (13.9)53.3 (47.8) | 10 (3.5) yrs | CV mortality (n=440)All-cause mortality (n=1,270) | HR, 1.81; 95% CI: 1.25–2.62HR, 2.73 95% CI: 1.71–4.36(Q1 [<20%min/h] versus Q5 [>88%min/h]) | Better HBDose–response |
| Azarbarzin et al. (2019)16 | SHHS (n=5,111)Population-based | 63.7 (10.9)47.2%men | 17.1 (15.3)46.6 (53.8) | 10.9 (3.1) yrs | CV mortality (n=313)All-cause mortality (n=1,142) | HR, 1.61; 95% CI: 1–2.6.1HR, 1.96; 95% CI: 1.11–3.43(Q1 [<16%min/h] versus Q5 [>71%min/h]) | Better HBDose–response |
| Blanchard et al. (2020)60 | Pays de la Loire Sleep Cohort (n=3,597)Clinical-based | 58 [48–67]63% men | 20 (IQR, 8–35) | 5.9 (IQR, 3.5–8.4) yrs | First incident stroke (n=83; 70 ischemic including TIA) | HR, 1.28; 95% CI: 1.05–1.57) | Better HBDose–response |
| Azarbarzin et al. (2020)62 | SHHS (n=4,881)Population-based | 63.6 (11.1)45.6%men | Men:11 (13.3)62 (64.7)Women:6.2 (10.1)37 (39.4) | 10.4 (3.4) yrs | Incident heart failure (n=543) | HR, 1.05; 95% CI: 0.95–1.17 | Better HB |
| Azarbarzin et al. (2020)62 | MrOS (n=2,653)Population-based | 76.2 (5.4)100% men | 11.4 (12.8)57.3 (53) | 8.8. (2.8) yrs | Incident heart failure (n=145) | HR, 1.22; 95% CI: 1.02–1.45 | Better HB |
| Kim et al. (2020)58 | Multi-Ethnic Study of Atherosclerosis (MESA) (n=2,055)Population-based | 68.4 (9.1)46%men | 14.8 (16.7)31.2 (41.7) | Cross-sectional | % change in SBP and DBP | SBP: 0.5 (−0.3 to 1.3); p=0.21DBP: 1.1 (0.4–1.8); p=0.002 | NA |
| Jackson et al. (2021)64 | Multi-Ethnic Study of Atherosclerosis (MESA) (n=1,895)Population-based | 68.2 (9.1)46% w | OSA, 79.2%56.5 (65.1) | Cross-sectional | Prevalent moderate-to-severe CKD | Prevalence rate, 1.36; 95% CI: 1.00–1.18)(Q1 [<16.24%min/h] versus Q5 [>84.4%min/h]) | Better HBDose–response |
| Trzepizur et al. (2021)51 | Pays de la Loire Sleep Cohort (n=5,358)Clinical-based | 60 (IQR: 51–69)63.7% men | 27 (IQR: 14–42) | 78 (IQR, 52–109) months | MACE (n=592) | HR, 1.21; 95% CI: 1.07–1.38 | Better HBDose–response |
Ref. [16]. Adjusted for age, BMI, race, gender (only in SHHS), sleep duration, smoking, alcohol (only in MrO2), non-CVD medical history, AHI, ODI, MinSat, TST90 and cardio-metabolic diseases.
Ref. [60]. Adjusted for age, gender, body mass index, alcohol intake, smoking status, diabetes, hypertension, history of cardiac disease and study site and CPAP adherence.
Ref. [62]. Adjusted for age, race, sex, interaction sex*HB, BMI, COPD, smoking, TST, diabetes, HTN, CHD, stroke.
Ref. [62]. Adjusted for age, race, BMI, COPD, smoking, TST, diabetes, HTN, CHD, stroke.
Ref. [58]. Adjusted for age, sex, race/ethnicity, body mass index (kg/m2), smoking status, cigarette pack-years, alcohol use, periodic limb movement, education level, odds ratio product and duty cycle. All results reported per SD increment of natural log-transformed hypoxic burden.
Ref. [64]. Adjusted for age, BMI, eGFR, hypertension, antihypertensives, diabetes mellitus, dyslipidemia.
8. Non-adjusted results.
Ref. [51]. Adjusted for: age, gender, body mass index, smoking status, presence of prevalent disease (diabetes, COPD and hypertension), type of sleep study, study site, beta blocker/calcium channel blocker medications and CPAP treatment.
HR: hazard ratio; IQR: interquartile range; Q: quintile; CKD: chronic kidney disease; AHI: apnea-hypopnea index; HB: hypoxic burden; SBP: systolic blood pressure; DBP: diastolic blood pressure; NA: no available; SHHS: Sleep Heart Health Study; MrOS: The Outcomes of Sleep Disorders in Older Men; CV: cardiovascular; TIA: transient ischemic attack; CHD: coronary heart disease; MACE: myocardial infarction, stroke, exacerbation of congestive heart failure, revascularization procedure (percutaneous coronary intervention, coronary artery bypass graft surgery) or all-cause death; OSA: obstructive sleep apnea defined as an AHI>5events/h.
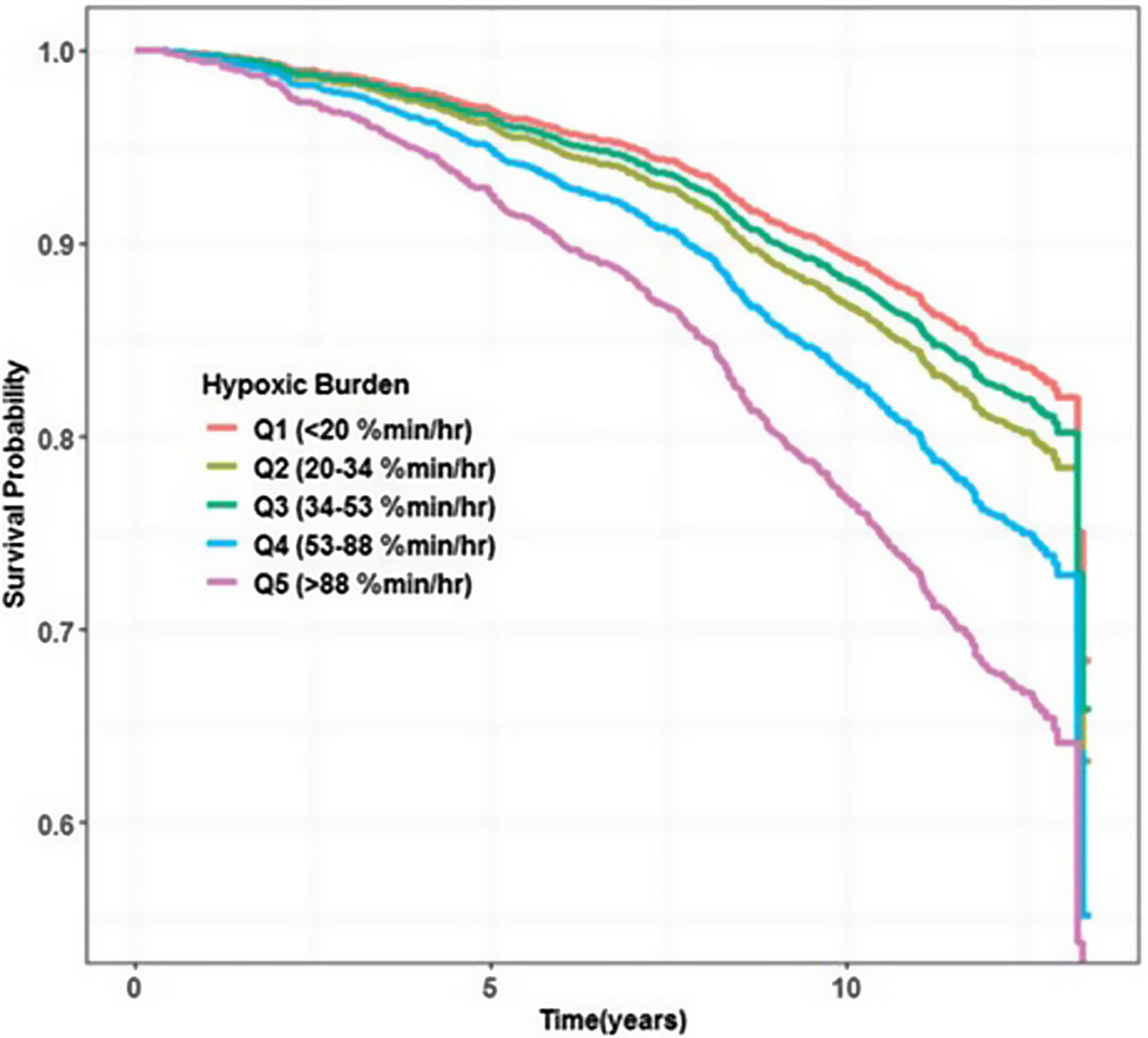
In view of these results and the aforementioned potential advantages of the HB over the standard desaturation indices, the first large study assessing this new metric addressed HB's relationship with cardiovascular mortality in 2019. Azarbarzin et al.16 using two large community-based cohort studies (Osteoporotic Fractures in Men Study [MrOS] and Sleep Heart Health Study [SHHS]) with 2743 men aged 76.3 (5.5) years and 5111 middle-aged and older adults (52.8% women) aged 63.7 (10.9) years, observed that unlike the AHI, the HB strongly predicted cardiovascular mortality. Individuals in the highest two HB quintiles presented a fully adjusted hazard ratio of 1.81 (95% CI: 1.25–2.62) and 2.73 (95% CI:1.71–4.36), respectively in the MrOS (Fig. 2). Those in the highest quintile in the SHHS had a hazard ratio of 1.95 (95% CI: 1.11–3.43). In MrOS, similar results were obtained when the main outcome was all-cause mortality. In summary, while HB was associated with excess cardiovascular mortality in both studies, the association with AHI was not observed in either of the two studies analyzed. On the other hand, ODI was only associated with mortality in the MrOS study, and the T90 predicted mortality only in the SHHS study with hazard ratios in both cases well below those observed for HB.
Hypoxic burden and incidence of major cardiovascular eventsThe only study analyzing the relationship between HB and major cardiovascular events was recently published by Trzepizur et al.51 This study separately examined the associations between the symptom subtypes described by Mazzoti et al. and others (“disturbed sleep group”; “minimally symptomatic group”, “excessive daytime sleepiness group” and “moderately sleepy group”) and HB and addressed incident cardiovascular diseases.52–54 A total of 5358 individuals with OSA (clinical cohort) without previous cardiovascular events (from the Pays de la Loire cohort) were included in this study. After a follow-up of 78 months (IQR: 52–109), 592 cardiovascular events (MACEs) were observed. In a fully adjusted model HB and T90 were the only significant predictors of MACE (HR 1.21, 95% CI [1.07–1.38] and 1.34 [1.16–1.55], respectively). It is worth noting for a clinical cohort the reported HB appeared to be underestimated, potentially explaining the lower Hazard ratio observed for HB compared to T90. Indeed, the median HB developed by Trzepizur et al.51 is ∼10% min/h lower than the HB calculated from a similar sample from the SHHS study despite having a significantly higher AHI (27 vs. 16events/h) and T90 (2.0 vs. 0.4% sleep time) than the SHHS study.55 It is unclear whether this difference was related to differences in analytical approach or other factors described previously. Despite this discrepancy, the study confirmed the prognostic capacity of HB (again stronger than AHI) not only in population-based studies but also in a clinical cohort of individuals with OSA. Limitations of this study include lack of information on confounding variables and linkage with health administrative data (unsupervised patient selection based on diagnostic codes).
Hypoxic burden and blood pressureOne of the most robust relationships observed with AHI in the cardiovascular sphere is between AHI and both prevalent and incident arterial hypertension,9 and especially with uncontrolled or resistant forms.30,56 In this relationship, there is agreement that the IH resulting from sleep-disordered breathing plays a fundamental role in raising blood pressure, especially during sleep.57
To assess whether HB also has prognostic value for hypertension, Kim et al.,58 using the Multi-Ethnic Study of Atherosclerosis (MESA) database of community-dwelling adults, included 1837 individuals with a mean age of 68.4 years and observed that in the overall series a higher overall HB was associated with a higher diastolic blood pressure (DBP) after adjusting for baseline covariates. For every 1 standard deviation (SD) increase in log-transformed HB there was a 0.9% increase in DBP (95% CI: 0.3–1.6, p=0.004) but there was no association with systolic blood pressure (SBP). The results were similar in both REM and NREM phases. However, among those not using anti-hypertensive drugs, every 1 SD increase in log-transformed HB was associated with a 1.1% increase in office SBP (95% CI: 0.1–2.1%) and 1.9% increase in office DBP (95% CI: 1.0–2.8%). In this case, differences were observed between HB in the REM and non-REM phases. Thus, higher REM-HB values were associated with higher SBP among those with mild OSA, and with higher DBP among never smokers. Finally, higher NREM-HB was associated with higher SBP, and both NREM and REM-HB were associated with higher DBP among non-medication users. Important limitations of this study were the cross-sectional nature of the study, inclusion of older subjects, and in-office measurement of BP rather than via 24h-ambulatory BP monitoring devices.
Hypoxic burden and strokeThe associations between the AHI and the incidence of stroke have been inconclusive, potentially due to variations in the mean age at the time of the sleep study, the primary or secondary nature of prevention, and/or the types of cerebrovascular events analyzed (hemorrhagic, transient ischemic attack [TIA] or ischemic stroke).59 Blanchard et al.60 examined the relationship between the HB and the incidence of new cerebrovascular events. A clinical database (Pays de la Loire Sleep Cohort) linked with data from the French administrative health was used (N=3597 followed for 5.9 (3.5–8.4) years). A total of 83 incident cerebrovascular event were observed (70 ischemic events). The fully-adjusted, log-transformed HB showed a higher prognostic value (HR 1.28 [95% CI: 1.05–1.57]) compared to other OSA severity measures, including AHI (HR 1.20 [95% CI: 0.93–1.55]), arousal index (HR 1.21 [95% CI: 0.86–1.71]), ODI (HR 1.13 [95% CI: 0.95–1.35]), and T90 (HR 1.06 [95% CI: 1.01–1.12]). In addition, a dose–response relationship between incident stroke and HB was observed. Finally, the association of HB with incident cerebrovascular events remained significant when hemorrhagic events were excluded (n=13) while the association became non-significant when TIAs (n=29) were excluded, potentially due to a significant decrease in statistical power. Interestingly, the association of HB and incident stroke did not appear to be affected by CPAP adherence (1159 had mean daily CPAP usage ≥ 4h). Stratified analyses demonstrated a stronger association between HB and incident stroke in non-obese patients and in those older than 60 years. Major limitations of this study include relatively small sample size (low number of incident events), residual confounding (not measured due to use of administrative health data), use of composite outcomes of different severity (TIA, stroke, cerebral hemorrhages), and the presence of CPAP-treated patients with good adherence.
Hypoxic burden and heart failureSimilar to stroke, the associations between OSA, quantified by the AHI, and heart failure (HF) have been inconclusive. Although several factors, including the severity of the HF (the worse the HF, the greater the number of central respiratory events) which proved to be more important than the number of obstructive events, age, and gender. In addition, the bidirectional relationship17 between AHI and HF severity may play a role. Despite these potential confounders, disease characterization, i.e. the use of the AHI, likely contributed to the observed inconsistencies in the literature.61
The association of HB and incident HF was examined using the data from the SHHS and MrOS studies.62 This study included 4881 middle-aged or older adults from the SHHS study (45.6% male with a mean follow-up of 10.4 (3.4) years and 543 incident HF), and 2653 (100% male with a mean follow-up of 8.8 (2.8) years and 145 incident HF) from the MrOS study. All individuals were free from HF at the time of the sleep study. Similar to other studies, HB was strongly associated with incident HF in men in both SHHS [HR, 1.18 (95% CI: 1.02–1.37)] and MrOS [HR, 1.22 (95% CI: 1.02–1.45)] cohorts, while the AHI was not. Excluding individuals with central sleep apnea or those with coronary heart disease at baseline did not meaningfully change these findings.18 Of note, in the SHHS, when HB analyzes were performed on all patients regardless of gender, HB was not significant in the fully adjusted model (HR 1.05, 95% CI: 0.95–1.17) nor was the AHI. However, the differences became evident when genders were analyzed separately. A potential reason for lack of association with incident HF in women could be lack of statistical power due to the small number of women with severe OSA, whether quantified by the AHI or HB (only 29.3% of women in SHHS had an AHI>30).
Finally, in a subgroup analysis, including men from both cohorts, the risk of incident HF was significantly higher in patients with high HB regardless of the AHI level (HR 1.36 [95% CI: 0.95–1.95] and 1.38 [95% CI: 1.10–1.74] in those patients with low and high AHI, respectively, but not in those patients with high AHI and low HB (adjusted HR 0.84 [95% CI: 0.47–1.51), providing additional evidence on the importance of the duration and depth of the desaturations as it relates to HF risk prediction.
Hypoxic burden and chronic kidney diseaseThe dose–response relationship between the prevalence of chronic kidney disease (CKD) and the severity of OSA (measured by AHI) was demonstrated in a meta-analysis that included 18 studies.63 Jackson et al.64 observed, in a cross-sectional study using the MESA cohort (n=1895), that HB was also associated with an increased prevalence of moderate-to-severe CKD defined as an eGFR<60mL/min/1.73m2 or albuminuria>30mg/g. Thus, participants with OSA showed a suggestive 20% higher prevalence ratio (PR, 1.20 [95% CI: 1.00–1.44]) for moderate-to-severe CKD. For participants in the highest versus lowest HB quintile, there was a 36% significantly higher moderate-to-severe CKD prevalence (PR, 1.36 [95% CI: 1.00–1.86]); likewise, the prevalence was significantly greater with higher HB measured as a continuous variable (PR, 1.06 [95% CI: 1.02–1.12]). Participants in the highest quintile of HB plus OSA had a 28% (PR, 1.28 [95% CI: 1.01–1.63]) increased prevalence of moderate-to-severe CKD. In all cases, the association with CKD was stronger for HB than for AHI. Finally, the dose–response relationship between HB and moderate-to-severe CKD was observed in the subgroups specified by race and ethnicity.
Relationship between hypoxic burden and other current and new sleep metricsAs described above, HB was designed to capture the frequency of respiratory events and the depth and duration of their ensuing desaturations. Therefore, by definition higher AHI commonly indicates a higher HB and vice versa. Previous population studies, including non-OSA and OSA populations reported an overall correlation coefficient of 0.8 for HB and AHI. However, in individuals with a moderate to severe OSA (AHI>15events/h) or severe OSA (AHI>30events/h), the correlation between AHI and HB was 0.66 and 0.51, respectively. The association between HB and other PSG parameters such as ODI, arousal index, wake time after sleep onset, and T90 were modest in general and were lowest in individuals with severe OSA.16 This would indicate that HB provides additional independent (and perhaps synergistic) information, which would allow, at least theoretically, to construct composite scores including HB. For example, despite a weak correlation between HB and T90, Trzepizur et al.51 observed that both HB and T90 were independent predictors of incident cardiovascular events in a clinical cohort of more than 5300 individuals with OSA. In another study by Kwon et al.,65 HB and the average lung to finger circulation time (LFCT; average time between the end of scored respiratory events and the nadir oxygen saturation) independently predicted CVD [1.36 (95% CI: 1.02–1.81)] and all-cause mortality [1.35 (95% CI: 1.14–1.60)]. These findings suggest that HB and LFCT, for example, separately capture CVD risk related to OSA (via HB) and other comorbidities, such as HF (via LFCT).
Finally, in some studies, HB and other novel predictors of CVD have been combined to improve risk prediction in OSA. For example, it has been recently shown that patients with coronary heart disease and higher pulse rate response to respiratory events (ΔHR) exhibit greater cardiovascular benefit from CPAP therapy.66 Azarbarzin et al.67 used the data from population-based MESA and SHHS studies to combine HB and ΔHR to improve CVD risk prediction. In the SHHS, individuals with a high ΔHR compared with a mid-range ΔHR were at increased risk of non-fatal or fatal CVD and all-cause mortality (non-fatal adjusted HR 1.60 (95% CI: 1.28–2.00); fatal adjusted HR 1.68 (95% CI: 1.22–2.30) and all- cause mortality adjusted HR 1.29 (95% CI: 1.07–1.55). The risk associated with a high ΔHR was particularly high in those with a higher HB (non-fatal, HR 1.93 [95% CI: 1.36–2.73]; fatal, HR 3.50 [95% CI: 2.15–5.71]; all-cause, HR 1.84 [95% CI: 1.40–2.40]). Once again, these results point to a synergistic relationship between HB and other novel OSA phenotypes (i.e. ΔHR).
ConclusionsThe current metrics to diagnose OSA and quantify its severity have important limitations. Therefore, alternative measures that better and more precisely risk stratify individuals with OSA are being developed. Among these measures, HB has shown promise in the community and in clinical cohorts over the last three years. In contrast to traditional metrics of OSA severity, HB consistently improved risk determination for both fatal and non-fatal cardiovascular events. In addition, the measurement of HB does not pose a challenge for either in-home or in-lab PSG systems,68–70 as it only requires recording of airflow and oxygen desaturation signals. Finally, a threshold of HB>60%min/h (i.e. 15min of 4% desaturations every hour) appears to identify patients who are at increased risk of cardiovascular morbidity and mortality. In theory, HB is also more sensitive to OSA treatment because it simultaneously captures three dimensions of OSA metrics (i.e. frequency, depth, and duration). Thus, a treatment that does not change the frequency of events (e.g. AHI), could potentially lower the depth and duration of desaturations which could potentially be adequate to lower the risk of adverse health outcomes.
Thus, we believe that, although future randomized clinical trials are needed to better assess its utility, going forward HB should play a role in clinical therapeutic decisions in OSA patients. This new measure could be progressively incorporated in sleep laboratories (as a first step in those reference sleep labs) for both clinical and scientific objectives. Doing so may allow for more appropriate decisions as to whom should be treated and how aggressive such treatment should be.
Future directionsThe following future directions should also be considered:
- 1.
Non-oximetric aspects of OSA: In addition to oxygen desaturation, cortical and/or autonomic arousals often follow respiratory events. Measurements, including the heart rate response to events (ΔHR),67 arousal intensity,71 sleep depth (“ORP”),72 and OSA-related symptoms can be potentially used to improve risk stratification.
- 2.
In Azarbarzin et al.16 original study, the impact of deep versus shallow desaturations were examined by giving a higher weight to deeper desaturations. This sensitivity analysis did not change the main findings; however, the impact of short/deep versus long/shallow desaturations can be systematically examined and their potentially prognostic values incorporated into future HB definition. In a number of small experimental studies, it has been postulated that mild intermittent hypoxia may be beneficial,73 however, in these studies, the cumulative daily exposure of mild intermittent hypoxia is substantially lower than that of OSA.74 In addition, future studies are needed to determine how factors, including baseline oxygen saturation, lung function, and obesity affect HB.
- 3.
All these metrics ultimately need to be tested prospectively in large observational or randomized controlled trials to better assess their utility in predicting risk and treatment effectiveness not only for cardiovascular diseases but also for other important outcomes including metabolic, cancer or neurocognitive outcomes as well as the response to OSA treatment.
Review design: MAMG. Data interpretation and writing the manuscript: All authors. All authors critically reviewed the manuscript, and approved its final submitted version.
FundingPhilips Healthcare without intervention in any part of the manuscript. MS-d-l-T has received financial support from a “Ramón y Cajal” grant (RYC2019-027831-I) from the “Ministerio de Ciencia e Innovación – Agencia Estatal de Investigación” co-funded by the European Social Fund (ESF)/“Investing in your future”. AA was supported by the American Heart Association (19CDA34660137), the National Institutes of Health (R01HL153874 and R01HL158765), and the American Academy of Sleep Medicine Foundation (188-SR-17). AA was partially supported by NHLBIR35HL135818.
Competing interestsAli Azarbarzin serves as a consultant for Apnimed, Somnifix, and Respicardia and receives grant funding from Somnifix outsite submitted work.