Classic cardiovascular risk factors do not explain all the cardiovascular events. Obstructive sleep apnoea (OSA) has been proposed as a potential and prevalent cardiovascular risk factor. Our study aimed to describe the prevalence of OSA in a middle-aged cohort with mild–moderate cardiovascular risk and evaluate its association with atherosclerotic disease.
MethodsThis is an observational cross-sectional ancillary study of the ILERVAS project which was aimed to study subclinical arterial disease in a cohort with mild–moderate cardiovascular risk. In a sample of consecutive subjects, we performed a sleep study and evaluate OSA prevalence and its association with carotid and femoral atheroma plaques and atherosclerotic burden.
ResultsOverall, 966 subjects with a median age of 57 years (25–75th percentile; 52–62) and a body mass index (BMI) of 28.5kg/m2 (25.6–31.6) were included. Of these, 72.6% (69.7%–75.3%) had OSA (apnoea–hypopnoea index (AHI)≥5/h); 35.7% (32.8%–38.8%) had mild OSA (AHI 5–14.9/h) and 36.9% (33.9%–39.9%) had moderate/severe OSA (AHI≥15/h). Mean oxygen saturation and the percentage of time with oxygen saturation<90% (CT90) were associated with atherosclerotic burden (eβ (95%CI) 0.932 (0.892, 0.974); 1.005 (1.002, 1.009), respectively) and total plaque (OR (95%CI) 0.88 (0.797,0.971); 1.013 (1.004,1.021), respectively). No association with the AHI or oxygen desaturation index was found.
ConclusionsThis study confirms a high prevalence of OSA in patients with mild–moderate cardiovascular risk and shows an association between atherosclerotic burden, total and femoral plaque with CT90 and mean oxygen saturation, suggesting the importance of OSA-related hypoxaemia in the induction of atherosclerotic disease.
Classic cardiovascular risk factors do not explain all the cardiovascular events that occur,1,2 and it is possible that other unknown cardiovascular risk factors may exist. In this sense, obstructive sleep apnoea (OSA) has been proposed as a new potential and prevalent cardiovascular risk factor.3,4
OSA is characterized by partial or complete episodes of collapse of the upper airway during sleep that cause intermittent hypoxia, hypercapnia, sleep fragmentation and exaggerated fluctuations in heart rhythm, blood pressure and intrathoracic pressure.5,6 In turn, these physiological disruptions can result in excessive daytime sleepiness, poor quality of life and cardiometabolic alterations.5,6
Several studies associated OSA to cardiovascular risk factors and support that OSA contributes to the pathogenesis of cardiovascular disease.3 Intermittent hypoxia and intrathoracic pressure swings have been associated with the initiation and progression of atherosclerosis.7,8 In contrast, some authors suggest that OSA may require other risk factors for the development of atherosclerotic plaques.9 Therefore, the impact of OSA on atherosclerosis and its interaction with other cardiovascular risk factors is not well known.
A higher prevalence of OSA has been described in individuals with cardiovascular conditions than in healthy adults10; furthermore OSA prevalence in cohorts with cardiovascular risk factors has triggered great interest.11
OSA and snoring have been associated with atherosclerotic plaque, especially in the carotid12,13; nevertheless, there are few studies with controversial data, and evidence is limited.
In this context, the current study aimed to describe the prevalence of OSA in a cohort of middle-aged subjects with mild–moderate cardiovascular risk and evaluate whether there is an association between polygraphy variables and the presence of atherosclerosis. Considering the controversial role of snoring on carotid but not femoral atherosclerosis, both territories were explored to assess potential differences.
MethodsDesign and Study PopulationThis is an ancillary study of the ILERVAS project (NCT03228459) aimed to evaluate subclinical arterial disease and hidden kidney disease in a cohort with mild–moderate cardiovascular risk.14 For more detailed information on the ILERVAS project see supplemental material.
The current study is an observational cross-sectional study of the ILERVAS cohort performed between March 2017 and September 2018. The aim is to analyze the prevalence of OSA in middle-aged subjects with mild–moderate cardiovascular risk and evaluate whether there is an association between OSA, the atherosclerotic burden and the presence of plaque in the different arterial territories. The ethics committee of our hospital approved the study (CEIC-1410), and all included subjects provided their informed consent at the inclusion.
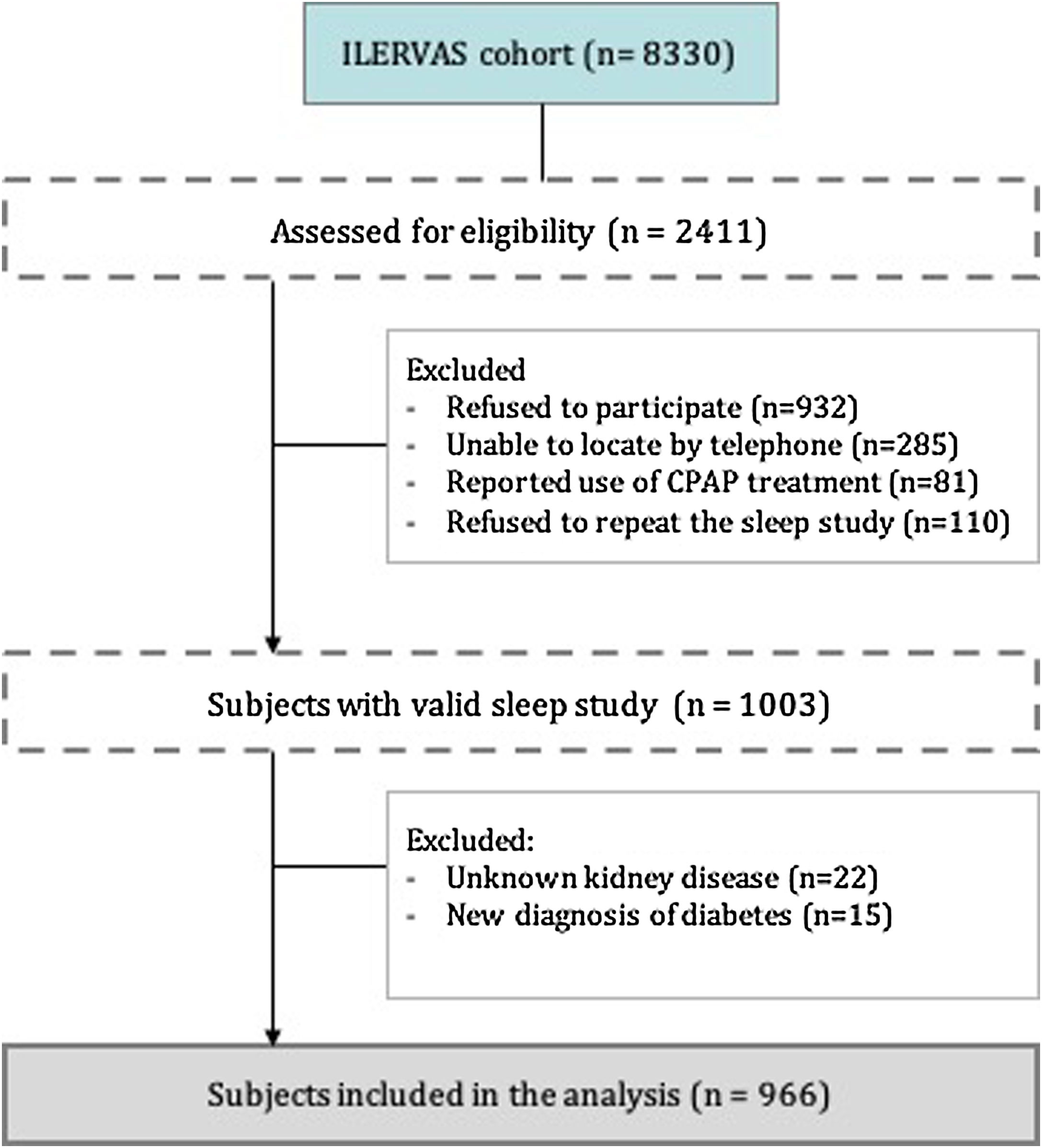
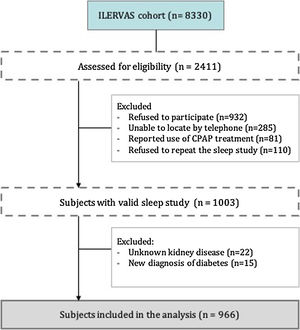
From the 8330 subjects included in the ILERVAS project, we assessed for eligibility 2.411 consecutive subjects who lived near Lleida city. From these subjects, 1408 were excluded, mostly refusal to participate or refusal to repeat invalid sleep study, remaining 1003 subjects with a valid sleep study. Finally, we included a total of 966 individuals who had not been treated previously for OSA and who had a valid sleep study (Fig. 1).
ProceduresSociodemographic and Clinical VariablesAt the inclusion visit on the ILERVAS project, age, sex, ethnicity, clinical history, comorbidities, anthropometric and clinical data were collected and vascular diagnosis tests were performed. Sleepiness was assessed by the Epworth Sleepiness Scale (ESS). Further detailed information about ILERVAS data collection is provided elsewhere.14
Sleep EvaluationAll the subjects included in the current study underwent one-night home cardiorespiratory polygraphy (ApneaLink Air device, Resmed, Sydney, Australia). Further information regarding the device characteristics and the scoring criteria used is provided in the supplemental material.
Based on the results in the sleep study, participants were classified as non-OSA (AHI<5events/h) or OSA (AHI≥5events/h). OSA subjects were classified as having mild (AHI 5–14.9events/h) or moderate to severe OSA (AHI≥15events/h).
Atherosclerotic Plaque EvaluationThe presence of atheroma plaques was evaluated by ultrasound in carotid and femoral territories following current guidelines14 and atherosclerotic burden was calculated. More details on ultrasound evaluation are provided in supplemental material.
Statistical AnalysisAs descriptive analyses, we compared sociodemographic characteristics and sleep parameters across categories of OSA severity groups. Data were described using the median (25–75th percentile) for continuous variables and percentages for categorical variables. The Shapiro–Wilk test was used to evaluate the normality distribution of the data. Differences between groups were assessed using the Kruskal–Wallis test for continuous variables and the Pearson chi-square test for categorical variables. The p for trend was evaluated using the Spearman rank correlation coefficient. The Agresti–Coull intervals were used to report 95% confidence intervals for the prevalence estimations.
The association between OSA severity groups and different cardiovascular risk factors were evaluated using multivariate logistic regression models. Two different adjustments were used. Multivariate logistic regression models were also used to explore the association between the presence of atherosclerotic plaques and the respiratory polygraphy parameters. Additionally, total plaque area was used to explore the association with respiratory polygraphy parameters using multivariate linear regression models. In that case, the distribution of the plaque area was not normal, and log transformation was performed. Models were adjusted using well-established confounding factors such as age, sex, current smoking habit, systolic blood pressure, diastolic blood pressure, total cholesterol, BMI, waist circumference, glycated haemoglobin (HbA1c), and antihypertensive and hypolipidemic treatment. All statistical analyses were conducted using R.15 All statistical tests were two-sided, and statistical significance was set at P-value<.05.
ResultsCohort CharacteristicsA total of 966 subjects were included (Fig. 1). The main characteristics of the subjects are shown in Table 1. Briefly, the median age was 57 years (25–75th percentile; 52.0–62.0), the gender ratio was near 1:1 and had a BMI of 28.5kg/m2 (25.6–31.6). The 82% of the included subjects presented 1 or 2 cardiovascular risk factors. At the time of inclusion, hypertension was identified in 39.3% of the subjects; moreover, 30.8% were obese, and 53.7% had dyslipidaemia. Overall, 6.0% had a family history of premature cardiovascular disease. Regarding atherosclerotic burden, the presence of plaque was observed in 70.3% of the subjects. Femoral plaque was the most frequently observed (55.2%).
Baseline Characteristics of the Cohort Stratified by the Severity of Obstructive Sleep Apnoea (N=966).
| Global N=966 | Non-OSA (AHI<5) N=265 | Mild OSA (AHI 5–14.9) N=345 | Moderate to Severe OSA (AHI≥15) N=356 | P for Trend | |
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Age (years) | 57.0 [52.0;62.0] | 56.0 [52.0;60.0] | 57.0 [52.0;62.0] | 58.0 [53.0;62.2] | .002 |
| Sex, female | 492 (50.9%) | 167 (63.0%) | 186 (53.9%) | 139 (39.0%) | <.001 |
| Tobacco habita | <.001 | ||||
| Non-smoking | 705 (73.0%) | 165 (62.3%) | 255 (73.9%) | 285 (80.1%) | |
| Smoking | 261 (27.0%) | 100 (37.7%) | 90 (26.1%) | 71 (19.9%) | |
| Anthropometric characteristics | |||||
| BMI (kg/m2) | 28.5 [25.6;31.6] | 26.3 [24.0;29.0] | 28.6 [25.7;31.5] | 30.0 [27.4;33.3] | <.001 |
| Waist circumference (cm) | 100 [93.0;108] | 94.0 [88.0;102] | 100 [94.0;107] | 104 [98.0;112] | <.001 |
| Male<102cm; Female<88cm | 303 (31.4%) | 111 (41.9%) | 99 (28.7%) | 93 (26.1%) | |
| Male≥102cm; Female≥88cm | 663 (68.6%) | 154 (58.1%) | 246 (71.3%) | 263 (73.9%) | |
| Neck circumference (cm) | 37.5 [34.5;41.0] | 35.0 [33.5;38.5] | 37.5 [35.0;41.0] | 40.0 [36.5;42.0] | <.001 |
| History of cardiovascular risk factors | |||||
| Hypertension | 380 (39.3%) | 68 (25.7%) | 138 (40.0%) | 174 (48.9%) | <.001 |
| Obesity | 298 (30.8%) | 42 (15.8%) | 106 (30.7%) | 150 (42.1%) | <.001 |
| Dyslipidaemia | 519 (53.7%) | 131 (49.4%) | 182 (52.8%) | 206 (57.9%) | .034 |
| FHCVD | 58 (6.0%) | 17 (6.4%) | 22 (6.4%) | 19 (5.3%) | .281 |
| Smoking | 406 (42.0%) | 138 (52.1%) | 146 (42.3%) | 122 (34.3%) | <.001 |
| Presence and area of atherosclerotic plaques | |||||
| Plaque total | 679 (70.3%) | 173 (65.3%) | 240 (69.6%) | 266 (74.7%) | .010 |
| Carotid | 468 (48.4%) | 115 (43.4%) | 160 (46.4%) | 193 (54.2%) | .006 |
| Femoral | 533 (55.2%) | 137 (51.7%) | 190 (55.1%) | 206 (57.9%) | .127 |
| Total area of plaques (cm2)b | 0.50 [0.23;1.04] | 0.50 [0.25;1.00] | 0.50 [0.24;1.01] | 0.48 [0.22;1.18] | .769 |
| Total area of carotid plaques (cm2)b | 0.22 [0.11;0.42] | 0.23 [0.10;0.47] | 0.22 [0.10;0.43] | 0.19 [0.11;0.39] | .858 |
| Total area of femoral plaques (cm2)b | 0.55 [0.25;0.95] | 0.51 [0.26;0.93] | 0.52 [0.25;0.84] | 0.58 [0.23;1.02] | .556 |
Results are presented as number of participants (%) and median [25th percentile; 75th percentile]. Definition of abbreviations: BMI=Body mass index; FHCVD: Familial history of cardiovascular disease; OSA=Obstructive sleep apnoea. The apnoea–hypopnoea index (AHI) is defined as the number of apnoea and hypopnoea events per hour of study. Non-OSA (AHI<5), Mild (AHI 5–15), Moderate to severe OSA (AHI≥15).
Globally, 72.6% (95% CI: 69.7%–75.3%) of the subjects had OSA (AHI≥5 events/h). Regarding OSA severity, 35.7% (32.8%–38.8%) of subjects had mild OSA (AHI 5–15events/h), and 36.9% (33.9%–39.9%) moderate to severe OSA (AHI≥15events/h). Of these, 14.9% had severe OSA (AHI≥30events/h).
OSA prevalence was slightly higher in men than in women (79.3% vs. 66.1%). Among moderate to severe OSA subjects, there was a high proportion of men and higher BMI, waist and neck circumference than those subjects with no or mild OSA (Table 1). Regarding sleep parameters, the median AHI was 10.3 (25–75th percentile; 4.5–20.5) events/hour. The median of the mean oxygen saturation was 93% (91%–94%), and the median of the percentage of time with an oxygen saturation<90% (CT90) was 8.0% (2.0%–27.0%). The median ESS score was 3.5 (2.0–5.0) (Table 2), only 3% of subjects presented an ESS score>10.
Sleep Characteristics of the Studied Cohort (N=966).
| Global N=966 | Non-OSA (AHI<5) N=265 | Mild OSA (AHI 5–14.9) N=345 | Moderate to Severe OSA (AHI≥15) N=356 | P for Trend | |
|---|---|---|---|---|---|
| Apnoea–hypopnoea index (events/h) | 10.3 [4.5;20.5] | 2.6 [1.4;3.9] | 9.0 [6.9;11.9] | 26.0 [19.3;40.2] | .000 |
| Apnoea index (events/h) | 1.50 [0.4;5.3] | 0.3 [0.0;0.9] | 1.3 [0.5;2.6] | 7.6 [2.7;15.4] | <.001 |
| Hypopnoea index (events/h) | 7.7 [3.4;14.7] | 1.8 [0.9;2.9] | 7.1 [5.2;9.6] | 17.9 [13.3;25.9] | <.001 |
| Mean O2 saturation % | 93.0 [91.0;94.0] | 94.0 [93.0;94.0] | 93.0 [92.0;94.0] | 92.0 [91.0;93.0] | <.001 |
| Minimum O2 saturation % | 83.0 [79.0;86.0] | 87.0 [83.0;89.0] | 83.0 [80.0;86.0] | 79.0 [73.0;82.2] | <.001 |
| CT90% | 8.0 [2.0;27.0] | 1.0 [0.0;6.0] | 6.0 [2.0;22.0] | 22.0 [8.8;41.0] | <.001 |
| Epworth Sleepiness Scale (ESS) scorea | 3.50 [2.00;5.00] | 3.00 [2.00;5.00] | 3.00 [2.00;5.00] | 4.00 [2.00;6.00] | .004 |
Results are presented as the median [25th percentile; 75th percentile]. Definition of abbreviations: CT90=percentage of time with an oxygen saturation<90%; OSA=Obstructive sleep apnoea. The apnoea–hypopnoea index (AHI) is defined as the number of apnoea and hypopnoea events per hour of study. Non-OSA (AHI<5), Mild (AHI 5–15), Moderate to severe OSA (AHI≥15).
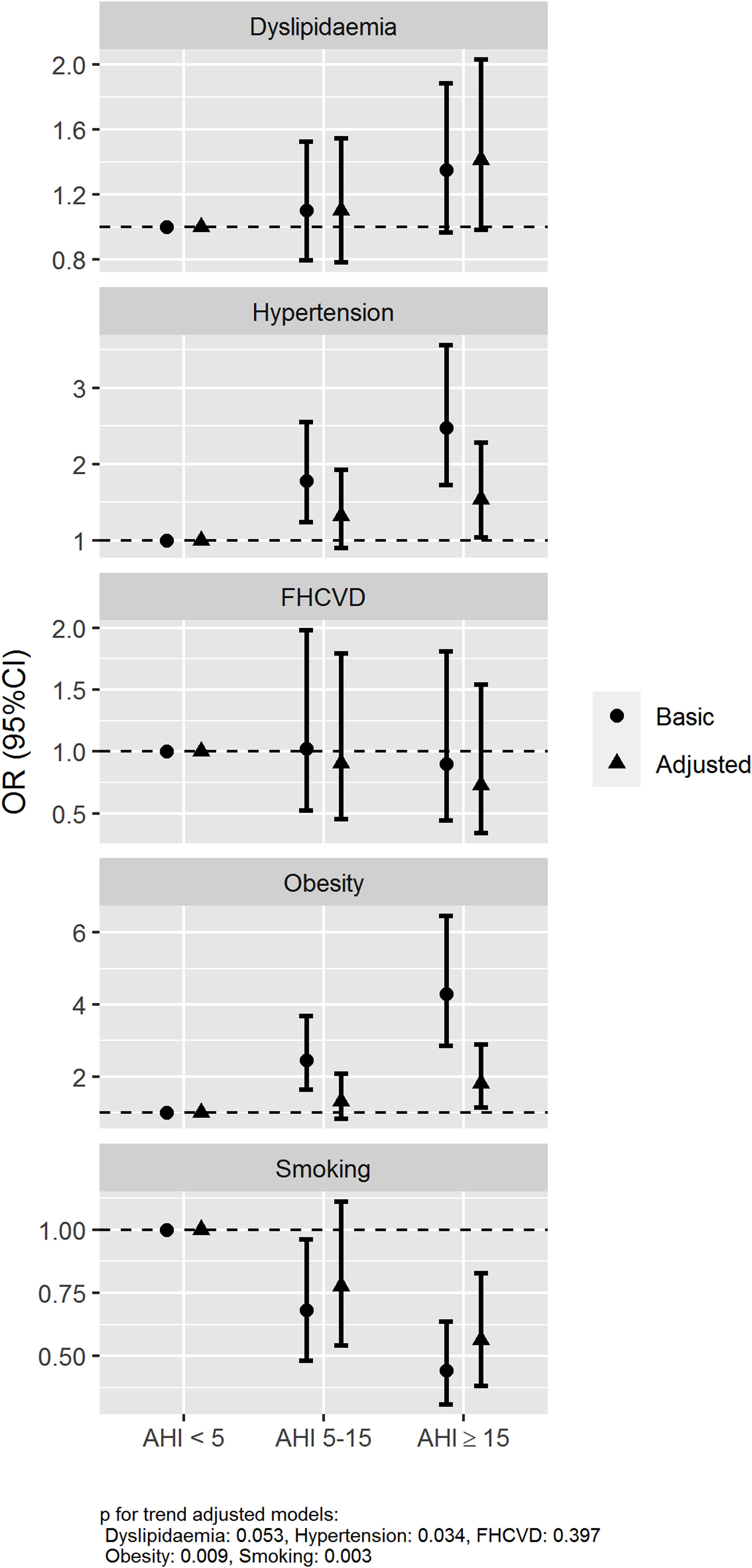
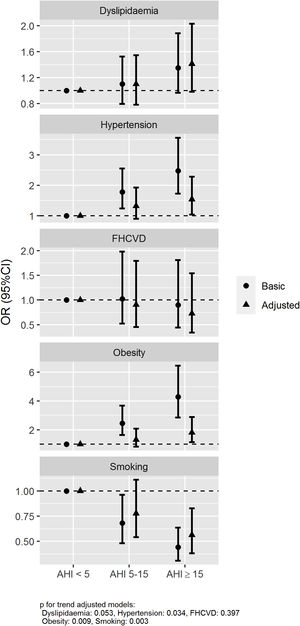
The association between the presence and severity of OSA and the history of hypertension, obesity, dyslipidaemia, smoking and family history of cardiovascular disease was assessed using logistic regression models with two adjustments, a basic model (age and sex) and an adjusted model (further adjusted for current smoking habit, BMI and neck circumference). The basic model showed a statistically significant association for AHI≥15/h vs AHI<5/h with a history of hypertension and obesity with an odds ratios (ORs) (95% confidence intervals) of 2.48 (1.72–3.56) and 4.29 (2.86–6.46), respectively. An association between OSA and history of smoking was also observed with an OR of 0.44 (0.31–0.64). Otherwise, no association between OSA and family history of cardiovascular disease and dyslipidaemia was observed, OR 0.89 (0.45–1.80) and 1.35 (0.97–1.88), respectively. These associations were also maintained in the adjusted model with further adjustment variables (Fig. 2).
Associated risk of OSA with history of hypertension, dyslipidaemia, obesity, smoking and family history of cardiovascular disease. Abbreviations: Family history of cardiovascular disease (FHCVD). Odds ratios (dots) and 95% confidence intervals (lines) are represented. The association between the presence and severity of OSA and the history of hypertension, obesity, dyslipidaemia, smoking and family history of cardiovascular disease was assessed using two logistic regression models: basic model (age and sex) and adjusted model (further adjusting by current smoking habit, BMI and neck circumference).
The association between the presence of atherosclerotic plaque and polygraphy variables was assessed using a multivariate logistic regression model. The presence of femoral and total plaque was associated with CT90 (OR (95% CI), 1.015 (1.008–1.023); 1.013 (1.004–1.021), respectively) and mean oxygen saturation (0.849 (0.775–0.931); 0.88 (0.797–0.971), respectively). No association was observed between these oximetric variables and the presence of carotid plaque. The AHI, oxygen desaturation index (ODI) and minimum oxygen saturation were not associated with the presence of plaque in any of the territories explored.
The association between polygraphy parameters and atherosclerotic burden was evaluated through multivariate regression linear models. As observed in the presence of plaque, an association between total and femoral atherosclerotic burden with CT90 and mean oxygen saturation was found, while no associations were observed with the AHI and ODI (Table 3).
Association Between Atherosclerotic Plaques and Sleep Parameters.
| Presence of Atherosclerotic Disease | ||||||
|---|---|---|---|---|---|---|
| Carotid Plaque (N=966) | Femoral Plaque (N=966) | Total Plaque (N=966) | ||||
| OR (95%CI) | P Value | OR (95%CI) | P Value | OR (95%CI) | P Value | |
| Apnoea–hypopnoea index (events/h) | 1.006 (0.997,1.015) | .197 | 0.997 (0.987,1.006) | .491 | 1.002 (0.992,1.013) | .677 |
| CT90% | 1.006 (0.999,1.013) | .061 | 1.015 (1.008,1.023) | <.001 | 1.013 (1.004,1.021) | .002 |
| Minimum O2 saturation % | 0.983 (0.963,1.004) | .111 | 0.985 (0.963, 1.007) | .179 | 0.977 (0.952,1.002) | 0.066 |
| Mean O2 saturation % | 0.925 (0.85,1.007) | .073 | 0.849 (0.775,0.931) | .001 | 0.88 (0.797,0.971) | .011 |
| Oxygen desaturation index 3% | 1.008 (0.998,1.017) | .106 | 0.998 (0.988,1.007) | .632 | 1.003 (0.992,1.014) | .599 |
| Total Plaque Area | ||||||
|---|---|---|---|---|---|---|
| Carotid Plaque Area (N=468) | Femoral Plaque Area (N=533) | Total Plaque Area (N=679) | ||||
| eβ (95%CI)a | P Value | eβ (95%CI)a | P Value | eβ (95%CI)a | P Value | |
| Apnoea–hypopnoea index (events/h) | 0.998 (0.993,1.003) | .537 | 1.001 (0.997, 1.006) | .546 | 0.999 (0.994, 1.004) | .649 |
| CT90% | 1.002 (0.998, 1.006) | .354 | 1.004 (1.001, 1.007) | .030 | 1.005 (1.002, 1.009) | .003 |
| Minimum O2 saturation % | 0.999 (0.987, 1.011) | .882 | 0.987 (0.977, 0.998) | .020 | 0.994 (0.982, 1.005) | .255 |
| Mean O2 saturation % | 0.979 (0.933, 1.027) | .388 | 0.945 (0.907, 0.984) | .007 | 0.932 (0.892, 0.974) | .002 |
| Oxygen desaturation index 3% | 0.999 (0.994, 1.004) | .748 | 1.002 (0.997, 1.006) | .504 | 0.9995 (0.995, 1.005) | .866 |
The association between the presence of atherosclerotic disease and the polygraph variables has been evaluated with a logistic regression model.
The association of the total plaque with the polygraphy variables has been evaluated using a linear regression model. Both models were adjusted by sex, age, current smoking habit, systolic blood pressure, diastolic blood pressure, total cholesterol, BMI, waist circumference, antihypertensive treatment, HbA1c, and hypolipidemic treatment. CT90=percentage of time with an oxygen saturation<90%; The apnoea–hypopnoea index (AHI) is defined as the number of apnoea and hypopnoea events per hour of study.
The present study confirms a high prevalence of OSA in subjects with mild–moderate cardiovascular risk and shows an association between the presence of atherosclerotic disease and parameters of nocturnal oxygen saturation.
To the best of our knowledge, our study is the first to evaluate the prevalence of OSA in a well-characterized cohort of middle-aged subjects with mild–moderate cardiovascular risk. Our findings show that the overall prevalence of OSA is 72.6%, being the prevalence of mild OSA 35.7%, and the prevalence of moderate to severe OSA 36.9%. OSA prevalence in our study is higher than previously reported in the general population16–19; nevertheless, it is difficult to compare among studies because of different types of sleep studies and different criteria to score and define OSA.
To date, a wide range of OSA prevalence has been reported, even when the same AHI cut-off point was used to define OSA. We observe that in a study performed in Hispanic subjects, the total prevalence of OSA was 25.8%, of which 9.8% for moderate OSA and 3.9% for severe OSA.18 Peppard et al.17 reported an estimated prevalence of OSA of 36.6% and 20.2% in men and women, respectively. Considering that age range and BMI were similar to our cohort, the prevalence observed in our study is higher than that described by Peppard et al.17 for both sexes. However, our prevalence is lower than reported by Heinzer.20 Differences could be explained by the use of polysomnography and the percentage of symptomatic subjects included in their study. Therefore, compared with studies using respiratory polygraphy, our data show a higher prevalence of OSA than previously reported in the general middle-aged population and suggest the importance of assessing OSA in subjects with at least one cardiovascular risk factor, even if they are asymptomatic.
An association of OSA with the risk of hypertension and obesity was found, as previously reported.21 Moreover, we observed a low probability of being a smoker with moderate to severe OSA. This result could be in line with previous studies concluding that the association between smoking and OSA has not been clearly stablished.22 Moreover, it is important to note that in our cohort, 42% of smoking patients present tobacco as the only risk factor while non-smoking patients were included in the study for presenting other cardiovascular risk factors and therefore we cannot exclude a selection bias that could have influenced the results.
Atherosclerotic burden, total and femoral plaque were associated with CT90 and mean oxygen saturation but not with AHI, minimal oxygen saturation or ODI. Our results are in line with previous studies linking mean oxygen saturation and CT90 with subclinical atherosclerosis.12,23–27 Unlike other studies,13,28,29 we did not find a relationship between AHI and the presence of plaque or the atherosclerotic burden in any territory. Our results are consistent with previous studies describing an association between atherosclerotic disease and OSA-related hypoxaemia parameters without an association with the AHI26 and indicate, as previously described, that atherosclerosis could be more strongly associated with hypoxaemia than with episodes of obstruction (represented by AHI).12,23,24 CT90 has been described as the strongest predictor of subclinical atherosclerosis25 and several observational studies showed that CT90 and mean SaO2 better predict cardiovascular mortality than the AHI.30,31
Moreover, our results are in line with recent publications indicating that the assessment of the severity of OSA only with the AHI could be insufficient as it measures the frequency of respiratory events but does not include key aspects of the deleterious effect of OSA on the cardiovascular system such as the duration and severity of the gasometrical changes. In this sense, Azarbarzin et al. have recently shown that the “hypoxic burden”, a new measure of OSA severity related to oxygenation changes associated with the respiratory events, could be more relevant to assess the relationship between OSA and cardiovascular disease than the AHI.32
It is important to note that CT90 can characterize not only nocturnal hypoxaemia associated with obstructive events but also persistent hypoxaemia that could be related to other conditions such as chronic respiratory diseases. In our cohort, CT90 values increase progressively as the severity of OSA (established by the AHI) increases. This fact suggests that CT90 reflects hypoxaemia related to OSA and not due to other underlying respiratory diseases such as COPD. Moreover, subjects included in the study presented spirometry values within the normal range. The mean CT90 value in our cohort was 8%. Even though it is not a high value, an association between CT90 and markers of atherosclerotic disease25 and CV mortality33 have been described with values of lower magnitude.
Differences between arterial territories were observed. An association between nocturnal hypoxaemia and atherosclerosis in femoral arteries was reported, while, no association between carotid territory plaques and any polygraphy variables was found. Our results are consistent with studies showing an association between OSA and peripheral arterial disease34 and are in line with previous studies not showing an association between OSA and carotid atheromatosis; territory in which controversy on the effect of OSA exists.28,35,36
Unlike our study, authors usually do not evaluate the presence of atherosclerosis in different territories and therefore evidence on the chronology and the territory mostly affected in OSA is scare. In studies assessing carotid and femoral territories in general population of similar characteristics than our patients, it has been described that femoral atherosclerosis could be an early marker of subclinical atherosclerosis37 and therefore it is possible that OSA related-hypoxia may have a greater impact on the territory with earlier affectation.
Multiple factors intervene in the generation of atherosclerosis and their interaction with OSA related hypoxaemia probably could influence differently the atherosclerotic process in carotid and femoral territories. The risk factors for atherosclerosis in the two areas (carotid and femoral) are similar,38,39 although peripheral arterial disease seems to be more dependent on smoking habits38 than carotid disease. Therefore, with the results, we can hypothesize that hypoxia may have a greater synergistic effect to promote atherosclerosis when interacting with the risk factors for peripheral arterial disease such as tobacco. In contrast with previous data,35 our results suggest that vibration produced by snoring may not have an important role in the generation of carotid plaque.
The main strength of our study is that it was performed in a large and well-characterized cohort of subjects with mild–moderate cardiovascular risk in which both arterial territories were evaluated.
Some limitations should be mentioned. First, the cross-sectional design limits causal inferences. Second, history of cardiovascular risk factors was not assessed at the same time than the sleep study and it could make associations less robust and difficult to establish causality, therefore the results should be extrapolated with caution. Third, subjects included are of a specific age group and the results cannot be extrapolated to other age groups. Additionally, age range was slightly different according to sex, making difficult to compare both groups. Forth, OSA diagnosis was performed using a simplified method; therefore, OSA prevalence can be underestimated and subjects misclassified. In addition, this simplified method does not provide information related to sleep structure, sleep fragmentation and arousal index, factors that have been related to atherosclerosis
ConclusionIn conclusion, our study shows a high prevalence of OSA in patients with mild–moderate cardiovascular risk. Moreover, the study shows an association between the atherosclerotic burden, total and femoral plaque with CT90 and mean oxygen saturation, suggesting the importance of OSA-related hypoxaemia in the induction of atherosclerotic disease.
Our data encourage clinicians to perform an energetic intervention to diagnose and control OSA in patients with cardiovascular risk factors. Future randomized clinical trials performed in well-characterized subjects according to their atheromatous risk are needed to evaluate if treating OSA could be beneficial and establish if subjects with subclinical arterial disease could be a target population.
Founding SourceThis work was supported by Diputació de Lleida, Instituto de Salud Carlos III [RETIC RD16/0009/0011]; Ministerio de Ciencia, Innovación y Universidades [IJC2018-037792-I]; Fondos recibidos por el ISCIII y fondos FEDER “Una manera de hacer Europa” [PI19/00907]; Oxigen Salud; Resmed; Jordi de Batlle acknowledges receiving financial support from Instituto de Salud Carlos III [ISCIII; Miguel Servet 2019: CP19/00108] and co-funded by European Regional Development Fund (ERDF)/European Social Fund (ESF), “Investing in your future”.
Conflict of InterestAll authors declare that there is no conflict of interest
The authors want to thank to IRBLleida Biobank (B.0000682) and PLATAFORMA BIOBANCOS PT17/0015/0027 for their technical support.
The authors of the manuscript want to thank to ILERVAS Group: Eva Castro-Boqué, Montserrat Martínez-Alonso, Dídac Mauricio, Pere Godoy, Manuel Sánchez-de-la-Torre, Manuel Portero-Otín, Eva Miquel, Marta Ortega, Jessica González, Silvia Barril, Mariona Jové, Marta Hernández, Ferran Rius Riu, Josep Franch-Nadal, Esmeralda Castelblanco, and Francisco Purroy.