Comparing sitting and supine vital capacity (VC) helps identifying diaphragm dysfunction.1 A 20–30% postural fall in VC (ΔVC) suggests bilateral diaphragm weakness,2–4 however, the ΔVC threshold differentiating the normal from the pathological is ill-established. A 15% ΔVC, chosen as representing twice the coefficient of variation of the measure in healthy subjects, has been proposed as the upper limit of normal,1 seems appropriate to identify bilateral diaphragm paralysis2 but may not be discriminatory enough to detect unilateral diaphragmatic paralysis.1–3 This may stem from other inspiratory muscles compensating for diaphragm dysfunction, hence a preserved inspiratory capacity (IC).5 The diaphragm, in addition to its inspiratory function, protects the lungs from compression by the abdominal content.6 This protective purpose is compromised by diaphragm atrophy,6 leading to infer that the abdominal content's cephalad movement when lying supine should reduce end-expiratory volume disproportionately in patients with diaphragmatic dysfunction, hence the corresponding reduction in expiratory reserve volume (ERV).7 Yet gravity-related postural reductions in end-expiratory volume also occur in normal individuals.8 Of note, standing upright involves applying a gravity-driven caudal force to abdominal viscera that could also modify lung volume and respiratory mechanics respective to their sitting values. Although it is usually accepted that the corresponding variations in lung volumes are trivial,9,10 forced expiratory volumes are frequently higher when standing.11 We therefore hypothesized that in normal individuals (1) sitting-to-supine postural variations in ERV, IC, or both (currently not described) would exceed, in percentage, the corresponding global VC changes; (2) standing-to-supine differences in VC, ERV and IC would deviate from sitting-supine such differences.
This study was approved by the Comité de Protection des Personnes Ile-de-France VI. Participants provided written consent. Inclusion criteria were: age over 18, non-obese, non-smoker, no history of respiratory, cardiac or neuromuscular disease, normal clinical examination. Lung function tests were performed according to the current recommendations and reference values of the European Respiratory Society10,12,13 in sitting, standing and supine positions in a random order (Fig. 1, Table 1) using a SpiroAir device running the Exp’air 1.28.20 software (Medisoft, Belgium). Total lung capacity (TLC) and residual volume (RV) were calculated using the measurement of functional residual capacity (FRC) using the Helium dilution technique13 and a slow VC maneuver with TLC equal to FRC plus IC and RV equal to FRC minus ERV. Maximal inspiratory (MIP) and expiratory (MEP) pressures and sniff nasal inspiratory pressure (SNIP) were measured while sitting,1,14,15 to rule out respiratory muscle dysfunction. Data are summarized as median and ([Q1; Q3]). A Friedman non-parametric test compared the three positions (p<0.05 for all volumes variations). Then sitting and standing positions were separately compared to the supine position using a Wilcoxon's signed rank test, giving access to ΔVCsit, ΔICsit and ΔERVsit (sitting-to-supine variations) and ΔVCsta, ΔICsta and ΔERVsta (standing-to-supine variations). The significance level was set to 0.05.
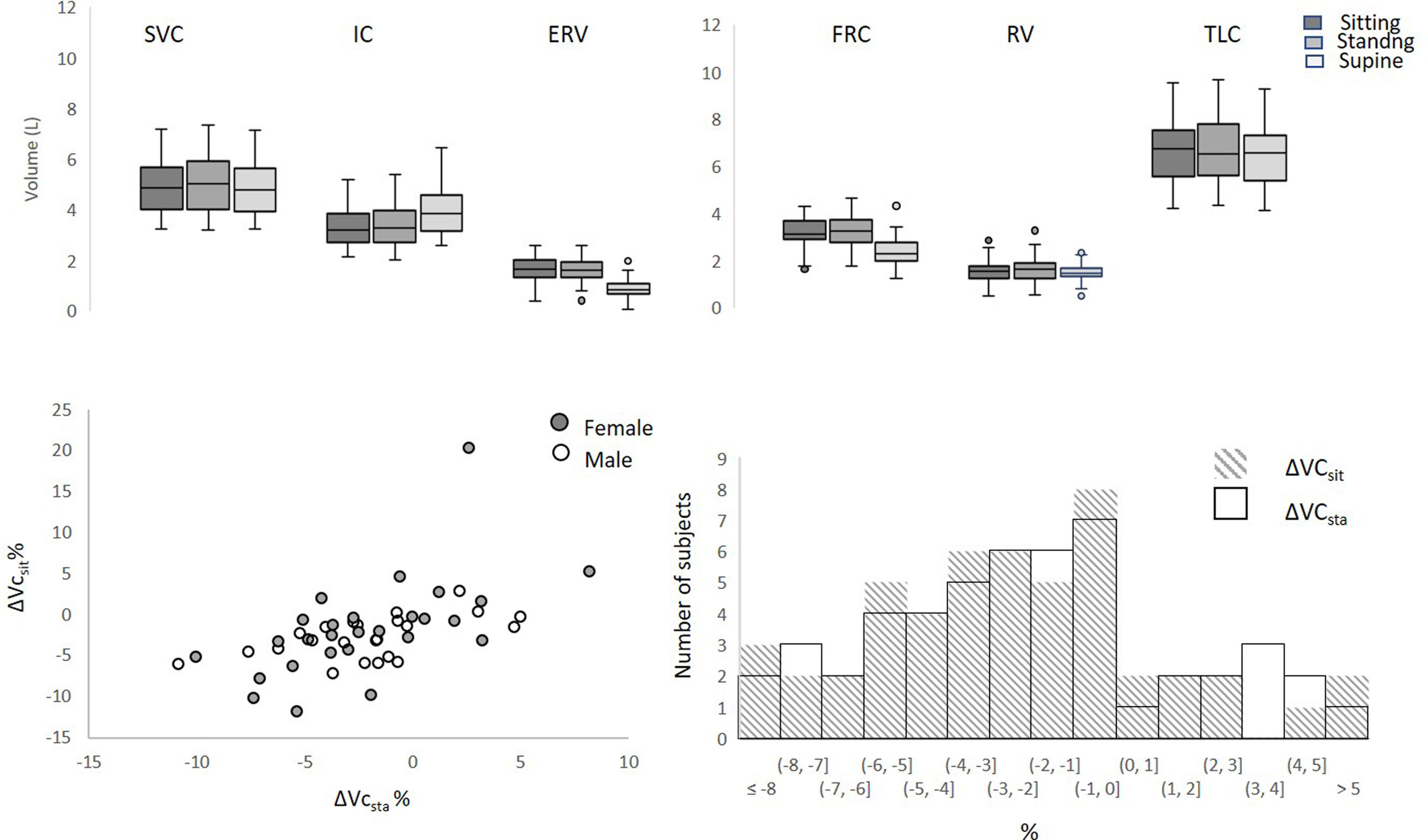
Lung volumes according to posture. Above: slow vital capacity; IC: inspiratory capacity; ERV: expiratory reserve volume; FRC: functional residual capacity; RV: residual volume; TLC: total lung capacity. p<0.05 Wilcoxon's signed rank test for all comparisons excepted for RV sitting-supine. Below: ΔVCsit: variation of vital capacity between supine and sitting positions; ΔVCsta: variation of vital capacity between supine and sitting positions; left: corresponding values of ΔVCsit and ΔVCsta by participant in females and males (standing–sitting −0.04L [−0.14; 0.05] in females and 0.03 [−0.13; 0.17] in males (ns, Mann–Whitney test); right: distribution of ΔVCsit and ΔVCsta per 1% class.
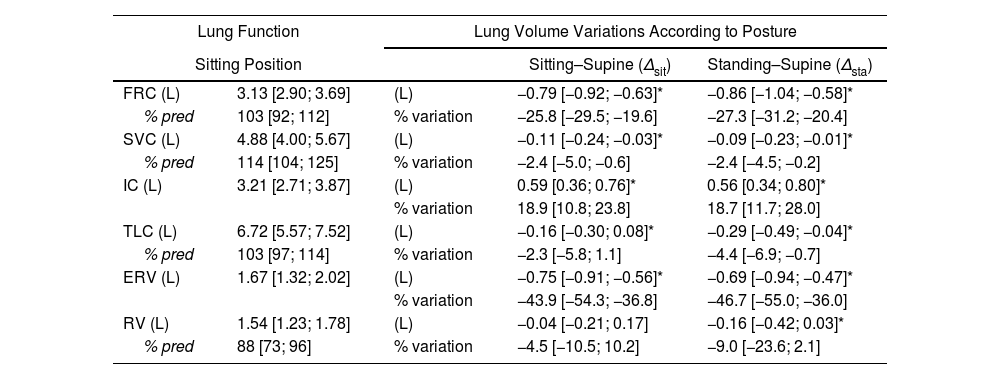
Lung Function and Lung Volume Variations According to Posture.
| Lung Function | Lung Volume Variations According to Posture | |||
|---|---|---|---|---|
| Sitting Position | Sitting–Supine (Δsit) | Standing–Supine (Δsta) | ||
| FRC (L) | 3.13 [2.90; 3.69] | (L) | −0.79 [−0.92; −0.63]* | −0.86 [−1.04; −0.58]* |
| % pred | 103 [92; 112] | % variation | −25.8 [−29.5; −19.6] | −27.3 [−31.2; −20.4] |
| SVC (L) | 4.88 [4.00; 5.67] | (L) | −0.11 [−0.24; −0.03]* | −0.09 [−0.23; −0.01]* |
| % pred | 114 [104; 125] | % variation | −2.4 [−5.0; −0.6] | −2.4 [−4.5; −0.2] |
| IC (L) | 3.21 [2.71; 3.87] | (L) | 0.59 [0.36; 0.76]* | 0.56 [0.34; 0.80]* |
| % variation | 18.9 [10.8; 23.8] | 18.7 [11.7; 28.0] | ||
| TLC (L) | 6.72 [5.57; 7.52] | (L) | −0.16 [−0.30; 0.08]* | −0.29 [−0.49; −0.04]* |
| % pred | 103 [97; 114] | % variation | −2.3 [−5.8; 1.1] | −4.4 [−6.9; −0.7] |
| ERV (L) | 1.67 [1.32; 2.02] | (L) | −0.75 [−0.91; −0.56]* | −0.69 [−0.94; −0.47]* |
| % variation | −43.9 [−54.3; −36.8] | −46.7 [−55.0; −36.0] | ||
| RV (L) | 1.54 [1.23; 1.78] | (L) | −0.04 [−0.21; 0.17] | −0.16 [−0.42; 0.03]* |
| % pred | 88 [73; 96] | % variation | −4.5 [−10.5; 10.2] | −9.0 [−23.6; 2.1] |
FRC: functional residual capacity; SVC: slow vital capacity; IC: inspiratory capacity; TLC: total lung capacity; ERV: expiratory reserve volume; RV: residual volume.
Fifty participants (34 years [26; 48]; 22 female, 172cm [165; 176]; 71kg [61; 79]; 24kg/m2 [21; 26]) were included and the complete dataset was analyzed (no missing data) (Table 1). All measured variables were within their normal range, including forced expiratory volume in 1s (FEV1) (3.84L [3.23; 4.54]; 109% predicted [101; 117]), FEV1/SVC (77% [71; 82]), MIP (104cm H2O [83; 128], 99% predicted [83; 121]), MEP (153cm H2O [129; 180], 80% predicted [68; 90]), SNIP (97cm H2O [79; 115], 94% predicted [87; 111]).
FRC significantly decreased by a median 25.8% (0.79L) from sitting to supine and 27.3% (0.86L) from standing to supine. VC also decreased significantly compared to sitting VC or standing VC (Table 1, Fig. 1), but ΔVCsit and ΔVCsta remained low, with a median reduction of −2.4% only for both and a maximal reduction never exceeding −12% (Fig. 1). None of the participants had both ΔVCsit and ΔVCsta exceeding −8% (Fig. 1). The conjunction of large FRC changes and small VC changes resulted in supine ERV and IC changes being noticeably more marked that their VC counterparts. Likewise, ERV significantly decreased in the supine position (median ΔERVsit −43.9%; median ΔERVsta −46.7%) while IC significantly increased (median ΔICsit 18.9%; median ΔICsta 18.7%). Expressed in volume, the IC increases exactly compensated the ERV decreases, explaining the stability of VC (median ΔERVsit+ΔICsit=−110mL to be compared with median ΔVCsit=−110ml; (median ΔERVsta+ΔICsta=−90mL to be compared with ΔVCsta=−90mL).
This study shows that lying supine markedly modifies the expiratory and inspiratory components of VC, in relationship with the postural decrease in FRC. Opposite ERV and IC changes result in a quasi-preserved VC. We observed a supine reduction in FRC of roughly 25%, amounting to an approximate median of 0.8L. This corresponds to observations made in healthy humans long ago4,16 and multiple times.8 We also confirmed that standing and sitting lung volumes were comparable (with individual differences) and that ΔVC was minimal between these two positions.8 The maximal ΔVC recorded in our participants was −12%, which is below the 15% threshold proposed to suspect an anomaly in the context of suspected diaphragm dysfunction.1 In addition, when ΔVCsta fell between −8% and −12%, ΔVCsit was always of −8% or less, and vice versa. This means that considering both ΔVCsta and ΔVCsit can bring the lower limit of normal ΔVC to −8%. Nevertheless, establishing a ΔVC threshold below the proposed 15% value1 may still not suffice to identify unilateral diaphragmatic palsy. In a study were ΔVCsit was tested against ultrasound indicators of diaphragm dysfunction, no single threshold of ΔVC could accurately predict unilateral diaphragm dysfunction.2
A thorough analysis of the ΔVC literature indicates that the differential effects of postural changes on the expiratory and inspiratory components of VC have not been described before in normal individuals. Our study, therefore, brings novel information in this respect. In our participants, the supine drops in FRC and the supine drop in ERV – of similar magnitudes – likely resulted from the gravity-related cephalad movement of the abdominal content displacing the diaphragm cranially despite its elasticity and tonic activity.6 Such a decrease in lung volume is associated with a deteriorated respiratory system impedance that proceeds from reduced lung compliance6,17,18 increased bronchial and upper airway resistance.9,11,19 Our participants were, however, able to maintain or near-maintain VC in the supine posture through compensatory increases in IC. Therefore, in their cases, the strength of the inspiratory muscles – diaphragm included – was sufficient to overcome the posture-related added inspiratory load. Such a finding is not surprising giving the large reserve that exists between the maximal inspiratory strength available and the strength necessary to bring normal lungs to their TLC.1,20 In summary, in supine normal humans, inspiratory muscle recruitment allows VC to be maintained despite the corresponding fall in FRC.
A study conducted in 13 patients with diaphragm dysfunction showed that the occurrence of orthopnoea coincided with that of expiratory flow limitation, leading the authors to consider the postural deterioration of respiratory mechanics as possibly causative of supine respiratory discomfort.7 In this study, the patients exhibited a median supine ERV reduction of 35%, similar to that we observed in our healthy population. However, the supine ERV reductions in the diaphragm weakness patients occurred on top of reduced sitting FRC values (leading some patients to exhibit a nearly zero ERV) that probably resulted from an atrophy-related loss of the barrier function of the diaphragm. In addition, contrary to what occurred in our participants, the diaphragm weakness patients did not exhibit compensatory changes in IC in the supine posture, probably because of a diaphragm weakness-related insurmountable load-capacity imbalance.
From the above, we submit that the value of supine spirometry to infer diaphragm dysfunction should be revisited and go beyond the mere determination of ΔVC. We acknowledge that this should be verified on a large cohort of patients with well documented diaphragmatic weakness.
Authors’ ContributionsConception and design of the study: VA, PL, CS, TS.
Data acquisition: VA, PL, RV, BS, CS, TS.
Analysis of the data: VA, PL, RV, BS, CS, TS.
VA, PL and TS drafted the initial manuscript. VA, PL, RV, BS, CS, TS contributed to the data interpretation and edited the manuscript for important scientific content. All the authors agree to be accountable for all aspects of the work in regard to accuracy and integrity.
Artificial Intelligence InvolvementNot applicable.
Funding of the ResearchThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.