Pleural effusion (PE) is a common yet complex disease that requires specialized, multidisciplinary management. Recent advances, novel diagnostic techniques, and innovative patient-centered therapeutic proposals have prompted an update of the current guidelines. This document provides recommendations and protocols based on a critical review of the literature on the epidemiology, etiology, diagnosis, prognosis, and new therapeutic options in PE, and addresses some cost-effectiveness issues related to the main types of PE.
Pleural effusion (PE) is a common clinical problem.1 The past decade has witnessed a gradual increase in the understanding of the pathophysiology, diagnosis, and use of imaging in this entity, and new pleural biopsy (PB) techniques and treatment options have emerged. This paper reviews the 2014 publication on the diagnosis and treatment of PE, provides guidelines based on a critical review of the literature, and updates previous recommendations.2
The manuscript was drawn up following a strict methodology, and recommendations were drafted to highlight the most relevant evidence using the GRADE methodology3 (Table 1). This paper highlights the most important aspects of the differential diagnosis of PE, and addresses the management of transudative PE, parapneumonic PE (PPPE), malignant PE (MPE), and tuberculous PE (TBPE). The complete document also includes other chapters: other PE of specific etiology (systemic diseases, abdominal-pelvic disease), and the diagnosis and treatment of very specific causes of PE, such as hemothorax, chylothorax and pseudochylothorax, and can be accessed online at https://www.separ.es/biblioteca.
Update of the Main Recommendations on Prevalence, Diagnosis, and Treatment in Pleural Effusion due to Different Etiologies.
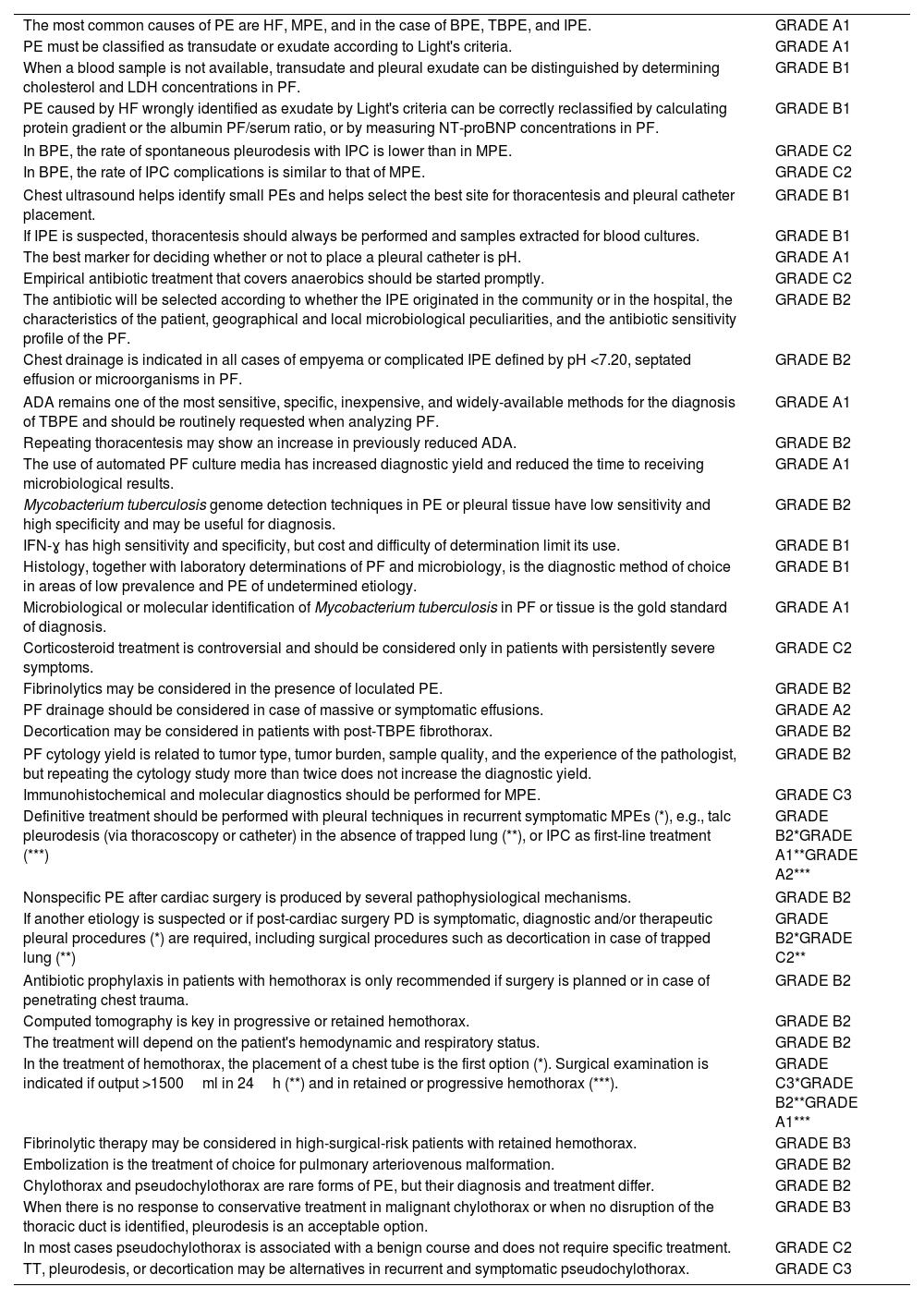
| The most common causes of PE are HF, MPE, and in the case of BPE, TBPE, and IPE. | GRADE A1 |
| PE must be classified as transudate or exudate according to Light's criteria. | GRADE A1 |
| When a blood sample is not available, transudate and pleural exudate can be distinguished by determining cholesterol and LDH concentrations in PF. | GRADE B1 |
| PE caused by HF wrongly identified as exudate by Light's criteria can be correctly reclassified by calculating protein gradient or the albumin PF/serum ratio, or by measuring NT-proBNP concentrations in PF. | GRADE B1 |
| In BPE, the rate of spontaneous pleurodesis with IPC is lower than in MPE. | GRADE C2 |
| In BPE, the rate of IPC complications is similar to that of MPE. | GRADE C2 |
| Chest ultrasound helps identify small PEs and helps select the best site for thoracentesis and pleural catheter placement. | GRADE B1 |
| If IPE is suspected, thoracentesis should always be performed and samples extracted for blood cultures. | GRADE B1 |
| The best marker for deciding whether or not to place a pleural catheter is pH. | GRADE A1 |
| Empirical antibiotic treatment that covers anaerobics should be started promptly. | GRADE C2 |
| The antibiotic will be selected according to whether the IPE originated in the community or in the hospital, the characteristics of the patient, geographical and local microbiological peculiarities, and the antibiotic sensitivity profile of the PF. | GRADE B2 |
| Chest drainage is indicated in all cases of empyema or complicated IPE defined by pH <7.20, septated effusion or microorganisms in PF. | GRADE B2 |
| ADA remains one of the most sensitive, specific, inexpensive, and widely-available methods for the diagnosis of TBPE and should be routinely requested when analyzing PF. | GRADE A1 |
| Repeating thoracentesis may show an increase in previously reduced ADA. | GRADE B2 |
| The use of automated PF culture media has increased diagnostic yield and reduced the time to receiving microbiological results. | GRADE A1 |
| Mycobacterium tuberculosis genome detection techniques in PE or pleural tissue have low sensitivity and high specificity and may be useful for diagnosis. | GRADE B2 |
| IFN-ɣ has high sensitivity and specificity, but cost and difficulty of determination limit its use. | GRADE B1 |
| Histology, together with laboratory determinations of PF and microbiology, is the diagnostic method of choice in areas of low prevalence and PE of undetermined etiology. | GRADE B1 |
| Microbiological or molecular identification of Mycobacterium tuberculosis in PF or tissue is the gold standard of diagnosis. | GRADE A1 |
| Corticosteroid treatment is controversial and should be considered only in patients with persistently severe symptoms. | GRADE C2 |
| Fibrinolytics may be considered in the presence of loculated PE. | GRADE B2 |
| PF drainage should be considered in case of massive or symptomatic effusions. | GRADE A2 |
| Decortication may be considered in patients with post-TBPE fibrothorax. | GRADE B2 |
| PF cytology yield is related to tumor type, tumor burden, sample quality, and the experience of the pathologist, but repeating the cytology study more than twice does not increase the diagnostic yield. | GRADE B2 |
| Immunohistochemical and molecular diagnostics should be performed for MPE. | GRADE C3 |
| Definitive treatment should be performed with pleural techniques in recurrent symptomatic MPEs (*), e.g., talc pleurodesis (via thoracoscopy or catheter) in the absence of trapped lung (**), or IPC as first-line treatment (***) | GRADE B2*GRADE A1**GRADE A2*** |
| Nonspecific PE after cardiac surgery is produced by several pathophysiological mechanisms. | GRADE B2 |
| If another etiology is suspected or if post-cardiac surgery PD is symptomatic, diagnostic and/or therapeutic pleural procedures (*) are required, including surgical procedures such as decortication in case of trapped lung (**) | GRADE B2*GRADE C2** |
| Antibiotic prophylaxis in patients with hemothorax is only recommended if surgery is planned or in case of penetrating chest trauma. | GRADE B2 |
| Computed tomography is key in progressive or retained hemothorax. | GRADE B2 |
| The treatment will depend on the patient's hemodynamic and respiratory status. | GRADE B2 |
| In the treatment of hemothorax, the placement of a chest tube is the first option (*). Surgical examination is indicated if output >1500ml in 24h (**) and in retained or progressive hemothorax (***). | GRADE C3*GRADE B2**GRADE A1*** |
| Fibrinolytic therapy may be considered in high-surgical-risk patients with retained hemothorax. | GRADE B3 |
| Embolization is the treatment of choice for pulmonary arteriovenous malformation. | GRADE B2 |
| Chylothorax and pseudochylothorax are rare forms of PE, but their diagnosis and treatment differ. | GRADE B2 |
| When there is no response to conservative treatment in malignant chylothorax or when no disruption of the thoracic duct is identified, pleurodesis is an acceptable option. | GRADE B3 |
| In most cases pseudochylothorax is associated with a benign course and does not require specific treatment. | GRADE C2 |
| TT, pleurodesis, or decortication may be alternatives in recurrent and symptomatic pseudochylothorax. | GRADE C3 |
ADA, adenosine deaminase; BPE, benign pleural effusion; HF, heart failure; INF-γ: interferon gamma; IPC, indwelling pleural catheter; IPE, infectious pleural effusion; LDH, lactate dehydrogenase; MPE, malignant pleural effusion; PE, pleural effusion; PF, pleural fluid; TBPE, tuberculous pleural effusion; TT, therapeutic thoracentesis.
The few epidemiological studies that have been published on PE are mostly retrospective, single-center, and based in Europe and the United States. The annual incidence of pleural diseases (PE and spontaneous pneumothorax) is estimated to be 350–360 cases per 100,000 inhabitants, and these entities are associated with high healthcare costs.4 Although there are more than 60 recognized causes of PE, 4 etiologies account for about 75% of cases: heart failure (HF), cancer, pneumonia, and tuberculosis, in order of frequency.5 It is important to emphasize that the formation of pleural fluid (PF) can sometimes be due to 2 or more concomitant diseases in a given patient.6 Another, much rarer phenomenon is bilateral PE with a different cause in each hemithorax, known in the literature as Contarini syndrome.7 Cancer is the second most frequent cause of PE,8 and although the prognosis of this diagnosis is poor, mortality associated with benign PE (BPE) is equally significant, as shown by a prospective study of 356 patients in which PE associated with cardiac, renal and hepatic diseases had a 1-year mortality rate of 50%, 46% and 25%, respectively.9
The first step in the etiological diagnosis of any PE is to distinguish between transudate and exudate.10,11 In spite of all the advances in recent years, transudates and exudates continue to be differentiated in clinical practice with the use of Light's criteria.12 These criteria have a sensitivity of 98% to identify exudates.12,13 However, about 25%–30% of transudates are wrongly classified as exudates. This is particularly common in patients taking diuretics or who have bloody PF.13 Approximately 80% of cardiac transudates incorrectly classified as exudates have a protein gradient >2.5g/dL, an albumin gradient >1.2g/dL, or pleural NT-proBNP concentrations >1500pg/mL. We can be more certain that the effusion is a transudate if both gradients are confirmed, the patient is >75 years, and the PE is bilateral.14 Hepatic hydrothorax (HH) is often misclassified as exudate. In these cases, a pleural fluid-to-serum albumin ratio of <0.6 would be a more sensitive parameter than the albumin gradient and would guide proper reclassification toward transudate.15 Other valid options may be PF cholesterol >55mg/dL or lactate dehydrogenase (LDH) >67% of the upper limit of normal serum LDH, parameters which both identify an exudate with a sensitivity of 97%.15
Transudative Pleural EffusionHeart failure is the most common cause of PE, and has a prevalence that may exceed 35% in some series.5,11 NT-proBNP in PF for the diagnosis of HF has a diagnostic sensitivity and specificity of 94% and 91%, respectively.16,17
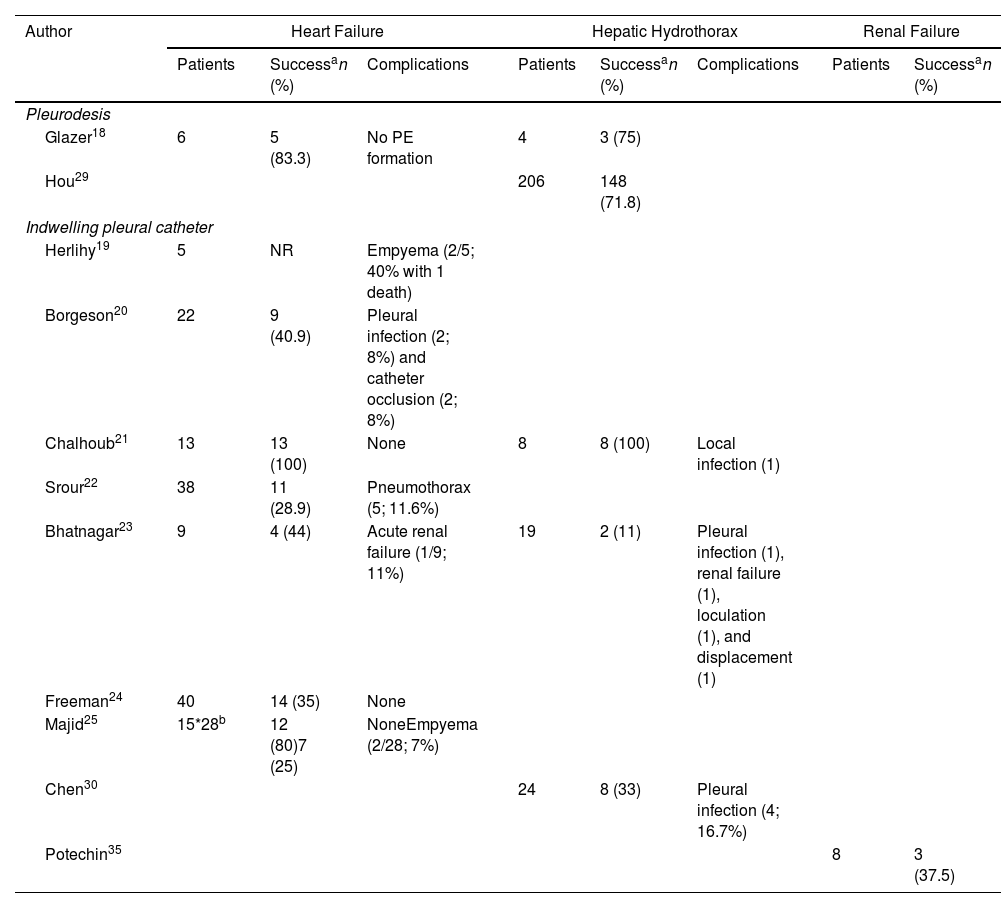
In general, PE will disappear in 89% of cases after 2 weeks of diuretic therapy, but it may be refractory in some patients.11 In such situations, therapeutic thoracentesis (TT) may be a satisfactory solution, but if more than 1–2 procedures per month are required, definitive options such as talc pleurodesis or indwelling pleural catheter (IPC)18–26 should be considered (Table 2).
Therapeutic Outcomes in Pleural Transudate.
| Author | Heart Failure | Hepatic Hydrothorax | Renal Failure | |||||
|---|---|---|---|---|---|---|---|---|
| Patients | Successan (%) | Complications | Patients | Successan (%) | Complications | Patients | Successan (%) | |
| Pleurodesis | ||||||||
| Glazer18 | 6 | 5 (83.3) | No PE formation | 4 | 3 (75) | |||
| Hou29 | 206 | 148 (71.8) | ||||||
| Indwelling pleural catheter | ||||||||
| Herlihy19 | 5 | NR | Empyema (2/5; 40% with 1 death) | |||||
| Borgeson20 | 22 | 9 (40.9) | Pleural infection (2; 8%) and catheter occlusion (2; 8%) | |||||
| Chalhoub21 | 13 | 13 (100) | None | 8 | 8 (100) | Local infection (1) | ||
| Srour22 | 38 | 11 (28.9) | Pneumothorax (5; 11.6%) | |||||
| Bhatnagar23 | 9 | 4 (44) | Acute renal failure (1/9; 11%) | 19 | 2 (11) | Pleural infection (1), renal failure (1), loculation (1), and displacement (1) | ||
| Freeman24 | 40 | 14 (35) | None | |||||
| Majid25 | 15*28b | 12 (80)7 (25) | NoneEmpyema (2/28; 7%) | |||||
| Chen30 | 24 | 8 (33) | Pleural infection (4; 16.7%) | |||||
| Potechin35 | 8 | 3 (37.5) | ||||||
NR, not reported.
HH is a rare complication of liver cirrhosis11,27 (Table 1 supplement). In general, the presence of HH is associated with a poor prognosis and a 1-year survival rate of 43%.27 The most frequent complication is spontaneous bacterial empyema that is observed in 15% of cirrhotic patients with PE28 (Table 1 supplement). Occasionally, the PF of patients with liver cirrhosis may be a chylothorax. If HH is refractory to optimized treatment, liver transplantation is the definitive treatment. If transplantation is contraindicated and PE recurs, an alternative is to implant a transjugular intrahepatic portosystemic shunt (TIPS) or perform surgical repair of the diaphragm. If the PE continues to recur, talc pleurodesis or the insertion of an IPC23,29,30 may be attempted (Table 2).
In a recent randomized clinical trial, in which patients with refractory transudative PE (HF, HH, or renal failure) were randomized to repeat TT procedures or IPC placement, neither approach offered any benefit in the control of dyspnea. Repeat TTs were associated with fewer complications, while patients treated with IPC underwent fewer invasive procedures, and complications were rare but severe.31
Inflammation of the pleural space can develop into trapped lung, a fibrous membrane in the visceral pleura that prevents lung expansion and increases negative pressure in the pleural space, producing PE.32 In this case, PF would in biochemical terms be a transudate, albeit at an early stage, in which the disease is still active and proteins may be in the range of exudates32 (Table 1 supplement). Several methods of predicting trapped lung have been proposed: pleural manometry, M-mode chest ultrasound, and the patient's symptoms during fluid withdrawal.33
A small percentage of MPEs behave as transudate, probably because PF builds up due to blocked lymphatic drainage. The application of predictive models that include clinical, radiological, and laboratory variables will help identify transudates which require cytology testing to rule out MPE.34
Transudates may also occur in less common situations, such as in patients receiving peritoneal dialysis who have also been treated with IPC35 (Table 2).
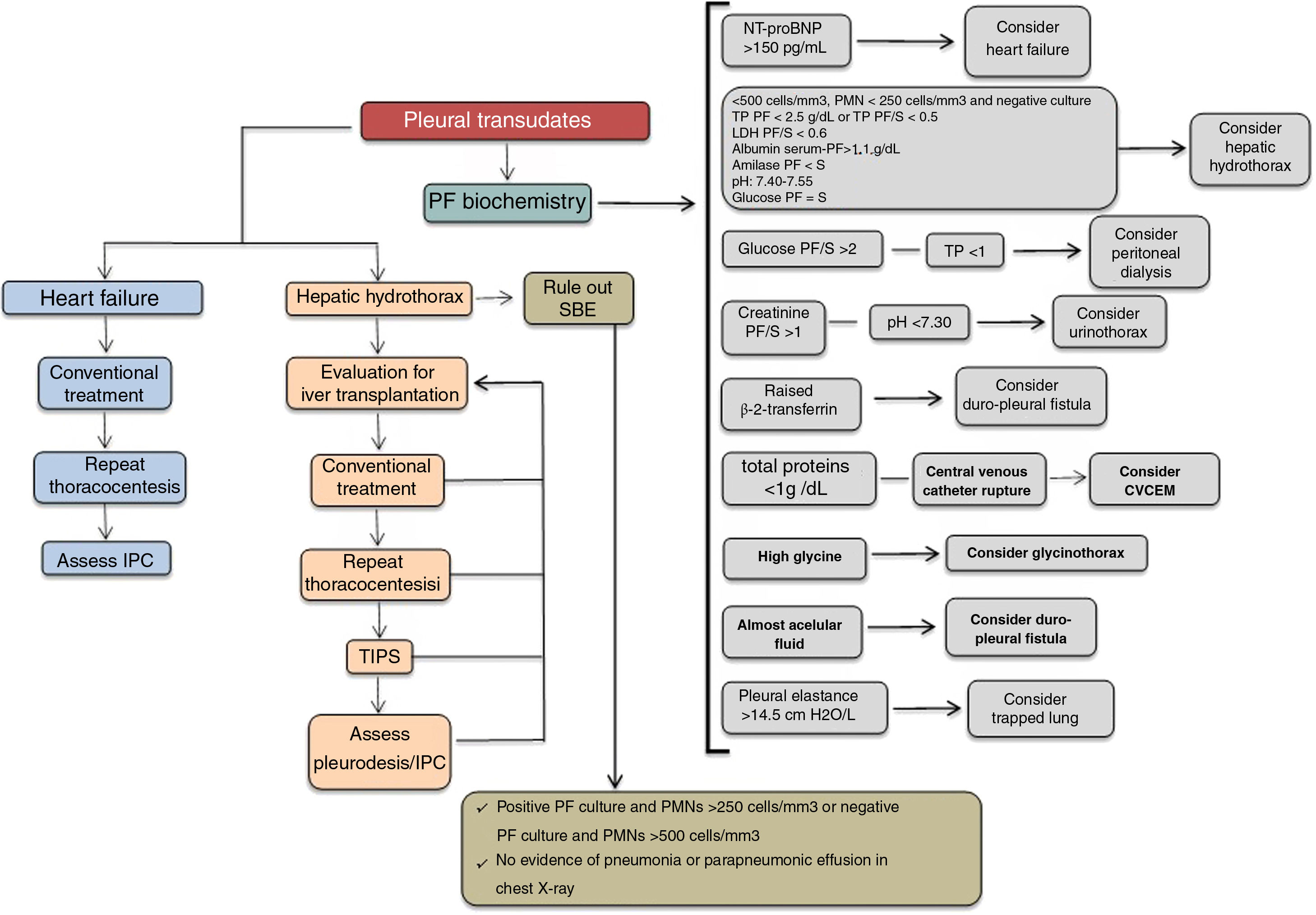
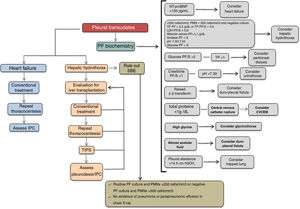
Fig. 1 shows an algorithm for managing pleural transudate.
Algorithm for the treatment of pleural transudate. CVCEM, central venous catheter extravascular migration; IPC, indwelling pleural catheter; LDH, lactate dehydrogenase; NT-proBNP, n-terminal pro-brain natriuretic peptide; PF, pleural fluid; PF/S, pleural fluid/serum ratio; PMN, polymorphonuclear; SBE, spontaneous bacterial empyema; TIPS, transjugular intrahepatic portosystemic shunt; TP, total proteins.
Parapneumonic pleural effusion (PPPE) is PE associated with lung infection, usually pneumonia, abscess, or infected bronchiectasis. When not associated with lung infection, it is called IPE (infectious PE). Up to 54% of pneumonias studied with chest ultrasound are associated with PPPE during their clinical course, and about 40% of these are complicated PPPE or empyema.36.37
Uncomplicated PPPE are PEs in the exudative phase and respond favorably to treatment with antibiotics alone. Complicated PPPEs are PEs in the fibrinopurulent phase, and present issues such as turbid PF with predominantly polymorphonuclear cells (PMNs) and microorganisms, pH <7.20, low glucose and high LHD, and the presence of fibrinous septa in the pleural space. These IPEs require not only antibiotic treatment but also chest drainage or surgery for resolution. Finally, organizing empyema, defined by the presence of pus in the pleural space, always needs to be drained. The RAPID clinical score based on baseline serum urea, patient age, PF purulence, source of infection, and serum albumin is useful for estimating the risk of death at 3 months and length of hospital stay in patients with PPPE, and for identifying PPPEs that may become complicated, but does not apply to empyema.38 More recently, the PILOT study showed that low-risk patients (RAPID score 0–2) had a 3-month mortality rate of 2.3%, medium-risk patients (RAPID score 3–4) 9.2%, and high-risk patients (RAPID score 5–7) 29.3%.39
The rate of isolation of microorganisms varies widely and can be improved with the incorporation of polymerase chain reaction techniques, PF inoculation of blood culture bottles (BACTEC), and pleural biopsy culture.40,41
Appropriate treatment of PPPE includes empiric antibiotics with anaerobic cover that must be started promptly and adjusted to microbiological test results.42 The antibiotic will be selected according to whether the PE was community-acquired or nosocomial, the characteristics of the patient, geographical and local microbiological peculiarities, and the antibiotic sensitivity profile of the PF. A third-generation cephalosporin combined with clindamycin or metronidazole or monotherapy with amoxicillin–clavulanic acid or piperacillin–tazobactam may be used. If the origin of the infection is nosocomial, recommended antibiotics include piperacillin–tazobactam or meropenem, and if the prevalence of methicillin-resistant Staphylococcus aureus of the hospital is ≥10%, vancomycin, linezolid, daptomycin or ceftobiprole or ceftaroline monotherapy should be added. Patients with beta-lactam allergy can be treated with tigecycline and aztreonam.42,43
Chest tubes are indicated in all cases of complicated empyema or PPPE. If pH is not available, glucose levels of less than 60mg/dL and LDH greater than 1000U/L are useful for identifying the need for pleural drainage. It should be noted that a pleural tube may be necessary in case of slow clinical progress, even if the pH is higher than 7.20. There is no consensus on the size of the most appropriate tube, but small catheters (10-14F) are easier to place, less traumatic, and more comfortable for the patient, and if they are combined with lavages and fibrinolytic therapy, their effectiveness is similar to that of larger caliber tubes.44 If septa are present in the pleural cavity and in the empyema, intrapleural enzyme treatment should be initiated early. Fibrinolytics rupture the fibrinous septa, facilitate the drainage of PF, and prevent the formation of septa in the pleural cavity. Streptokinase, urokinase or alteplase are used mainly, but there is no consensus on the doses to be used. There is insufficient scientific evidence to recommend any particular agent or dose over another.44 In patients with complicated PE or empyema, intrapleural fibrinolytic therapy was associated with a reduced need for surgery and less overall treatment failure, but no impact on mortality was observed.45 DNase is an enzyme that reduces the viscosity of pus and improves the drainage of very dense fluids, so it may be indicated from the outset or after failure of simple chest drainage.46
Other interventions, such as medical thoracoscopy, can be used to mechanically rupture pleural adhesions and to place chest tubes under direct vision.47 In observational studies, this technique demonstrated good therapeutic efficacy in PPPE and empyema when performed as a first-line procedure or if chest drainage fails, so it is extremely promising in experienced centers, particularly in frail elderly patients with comorbidities in whom surgical options may be contraindicated.47
Surgery, specifically video-assisted thoracoscopy, is an option for patients with septated effusion resistant to medical treatment. It can be used for pleural debridement with subsequent lung reexpansion and helps control sepsis.48 In the case of highly organized empyema with extensive pleural fibrosis, decortication may be necessary.
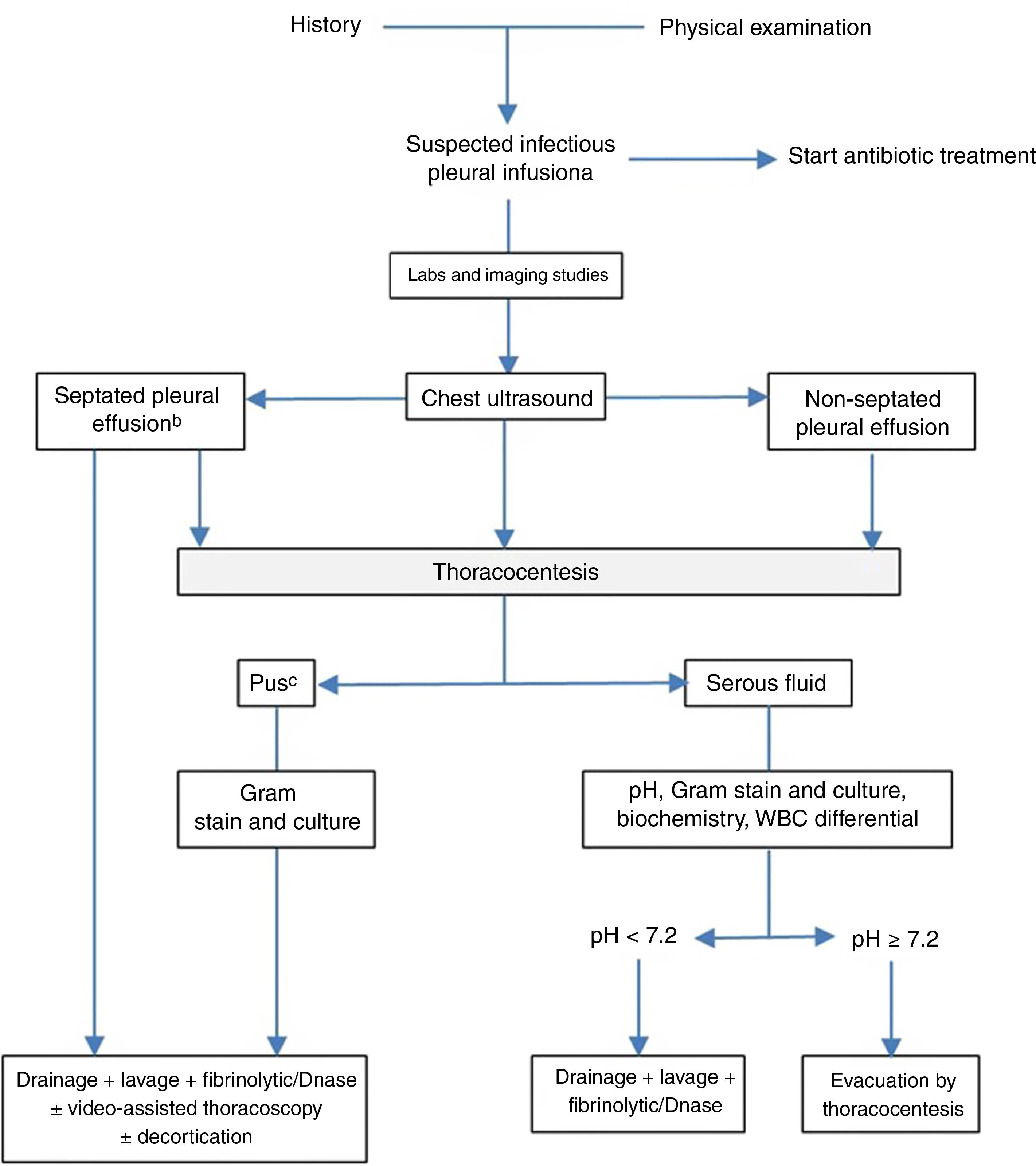
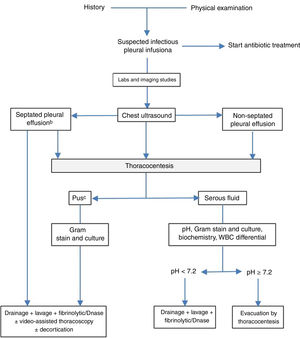
Fig. 2 shows an algorithm for treating PPPE.
Algorithm for the treatment of parapneumonic effusion. aIn all cases, empirical antibiotic treatment should be started promptly, and then adjusted according to the results of the cultures. Other indications of drainage are the existence of bronchopleural or pleuroparietal fistula, air-fluid level, large volume or sepsis. bThe presence of septa may also be based on CT scan or chest X-ray findings. cAlso if the pleural fluid is cloudy or foul-smelling.
Along with nodal involvement, the most common extrapulmonary manifestation of tuberculosis is TBPE. It is estimated to occur in 10%–19% of tuberculosis cases in Spain.2,5 Typically, TBPE is an exudate with a nucleated cell count ranging in most cases from 1000 to 6000cells/mm, usually with a predominantly lymphocytic content of >50% (sometimes even >90%) or a lymphocyte/neutrophil ratio >0.75. Patients who are in the early phase of the disease (less than 2 weeks) or who have tuberculous empyema may have predominant PMNs (between 5% and 17% of cases). The finding of mesothelial cells >5% and eosinophilia >10% is exceptional.49
Adenosine deaminase (ADA) remains the primary biomarker for TBPE49 with an overall sensitivity of 92% and a specificity of 90%.50 ADA has 2 isoforms: ADA1 and ADA2, and although ADA2 represents 88% of the total activity and is the predominant isoform in TBPE with a sensitivity and specificity of 97% and 94%, respectively, determination of ADA is recommended in clinical practice, as it is also a highly sensitive, low-cost and rapidly available option in biochemical analyses routinely performed on PF.51 In areas of high TB prevalence, PE containing predominantly lymphocytes associated with ADA >40U/L has a positive predictive value of 98% and provides sufficient evidence to start anti-tuberculosis treatment.51,52
A number of diseases can increase ADA levels in PF, including PPPEs, lymphomas, some solid tumors, and systemic rheumatic diseases (especially rheumatoid arthritis and systemic lupus erythematosus), as well as other rarer infectious diseases such as brucellosis, Q fever, histoplasmosis, or coccidiomycosis. Despite a fixed cut-off, levels above 250U/L suggest diagnoses other than TBPE.53
Interferon gamma (INF-γ) is a key cytokine in the immunopathogenesis of mycobacterial infection. It is released by activated CD4 lymphocytes and it primarily activates macrophages to increase their anti-mycobacterial activity. Concentrations may sometimes increase in blood cancers and empyema. The sensitivity and specificity of INF-γ are excellent for the diagnosis of TBPE, with some studies reporting values of above 85% and 95%, respectively.54
The tests known as INF-γ release assays (IGRAS) detect the release of INF-γ by sensitized T cells in peripheral blood or PF in response to specific antigens of encoded mycobacteria in the genome region known as the region of difference 1. Its sensitivity and specificity in PE appear to be similar to those of blood, and it has a sensitivity and specificity of 77% and 71% in tissue samples, and 78% and 72% in PE, respectively.55
Lysozyme, an enzyme released by the bactericidal activity of macrophages and PMNs, may be raised in TBPE, but also occurs in empyema or MPE. The PF to serum lysozyme ratio appears to be useful in the diagnosis of TBPE if empyema is ruled out.49
In order to avoid further testing, the combination of parameters has also been shown to improve the diagnosis of TBPE.56–58
In terms of microbiology, to be positive, a conventional sputum smear in PF requires a density of acid-alcohol-fast bacilli >10,000/ml, so this test has a low yield (<10%).59 Solid culture media have a low mycobacteria growth rate (<30%), while semi-automatic liquid culture media have higher yields in immunocompetent patients and in HIV patients: 56% and 75%, respectively. The use of polymerase chain reaction techniques has improved the cost-effectiveness of microbiological studies. Combining different samples also improves microbiological yield by up to 80%.49
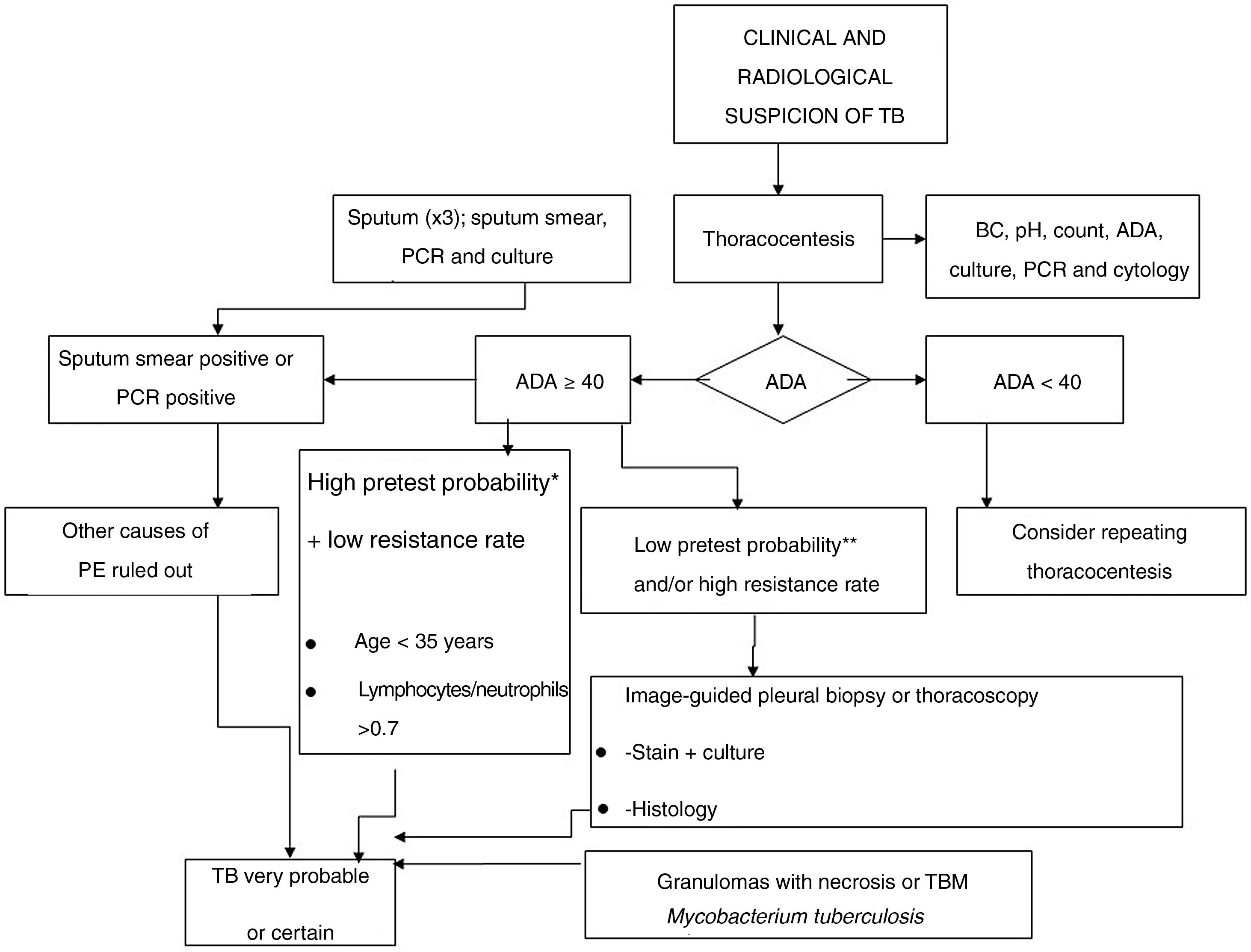
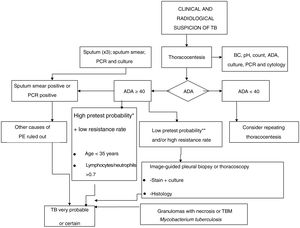
A diagnosis of probability is established in cases with high suspicion of tuberculosis, or in endemic areas when a lymphocyte/neutrophil ratio >0.75 and elevated ADA are detected in PE, or in cases where the presence of calcified granulomas is demonstrated in a PB. The definitive diagnosis of TBPE is based on the demonstration of Mycobacterium tuberculosis in sputum, PF or PB samples.49,60Fig. 3 shows the diagnostic algorithm for PPPE.
In spite of the good response to anti-tuberculosis treatment, there are cases in which progress can be poor and complications such as tuberculous empyema can develop, requiring surgical treatment.61 Another complication is residual diffuse pleural fibrosis or fibrothorax. If the patient is symptomatic, decortication may be performed.61 Pleural drainage and the administration of fibrinolytics may be a therapeutic alternative.62 In large effusions, TT provides symptomatic relief and appears to be associated with a decrease in the incidence of residual pleural thickening.
Hydropnemothorax may occasionally occur due to pleural rupture of a parenchymal cavitary lesion, and may become chronic and require chest drainage or surgery.61.62
Standard first-line treatment regimen is the same as for pulmonary tuberculosis, but there are considerations with regard to other treatments. There is insufficient evidence to support the indication of anti-inflammatories, such as corticosteroids.63,64 In general, their use should be avoided, but an exception may be made in patients who remain very symptomatic after weeks of treatment.
Fig. 3 shows the diagnostic algorithm for PPPE.
Malignant Pleural EffusionMPE is the result of direct infiltration of the pleura by neoplastic cells. Paramalignant PE is associated with the primary tumor but the cancer cells do not directly infiltrate the pleura.65
The most common cause of MPE is metastatic disease, for example lung and breast carcinomas, which account for up to 75% of all MPEs.65 Mesothelioma accounts for 10%.66
Contrast-enhanced computed tomography (CT) is the imaging technique of choice. There are some CT findings that may guide us toward the neoplastic origin of the effusion, such as circumferential thickening of the pleural surface, nodular thickening of the pleural surface, involvement of the mediastinal pleura, or evidence of a primary tumor. These findings show a sensitivity of over 88%.67
The definitive diagnosis of MPE is based on the demonstration of neoplastic cells in fluid and/or pleural tissue.
The diagnostic yield of PF cytology ranges from 49% to 91%.68,69 Repeating the cytological study more than twice does not improve the yield, and the addition of biopsy sampling is recommended when a second cytology is required.8,68
Ultrasound-guided PB is especially indicated in the presence of pleural thickening or masses. Reported sensitivity ranges from 76% to 93%, with a specificity of 100%.70 CT-guided PB can also be performed, offering a yield similar to that of ultrasound.71
Thoracoscopy PB is the gold standard in the diagnosis of MPE, and has a diagnostic yield of between 90% and 100%.72 It can be performed under general anesthesia in the operating room (surgical thoracoscopy) or under deep sedation and local anesthesia in the respiratory endoscopy room (medical thoracoscopy). Medical thoracoscopy can be either rigid or semi-rigid. Both approaches have similar diagnostic yield, similar complications,73 and a sensitivity of approximately 95%.73
A diagnosis of MPE confers high morbidity and mortality.8 Many prognostic models have been proposed that could assist clinical decision-making, but only one has been externally validated, namely, the LENT prognostic stratification scale based on 4 parameters: LDH levels in PF, the ECOG scale, the serum neutrophil/lymphocyte ratio, and tumor type.74
The main objective of the treatment of MPE is to reduce symptoms and improve quality of life. The current recommendation is to offer early definitive treatment for the control of MPE and not wait for the effect of targeted therapies.75,76
TT is not recommended as the only therapeutic measure, except in patients with a short life expectancy (less than 1 month). In the absence of trapped lung, pleurodesis is preferable, with talc being the agent of choice. The success of pleurodesis is not affected by the form of administration (via chest tube or thoracoscopy), and the rate of complications and improvement in symptoms are also similar with both methods.77,78
IPC is useful in cases where pleurodesis is contraindicated or has failed, and in the case of trapped lung and/or poor functional status.79 This is also now a first-line option that allows the therapeutic process to be moved to the outpatient setting.80–82
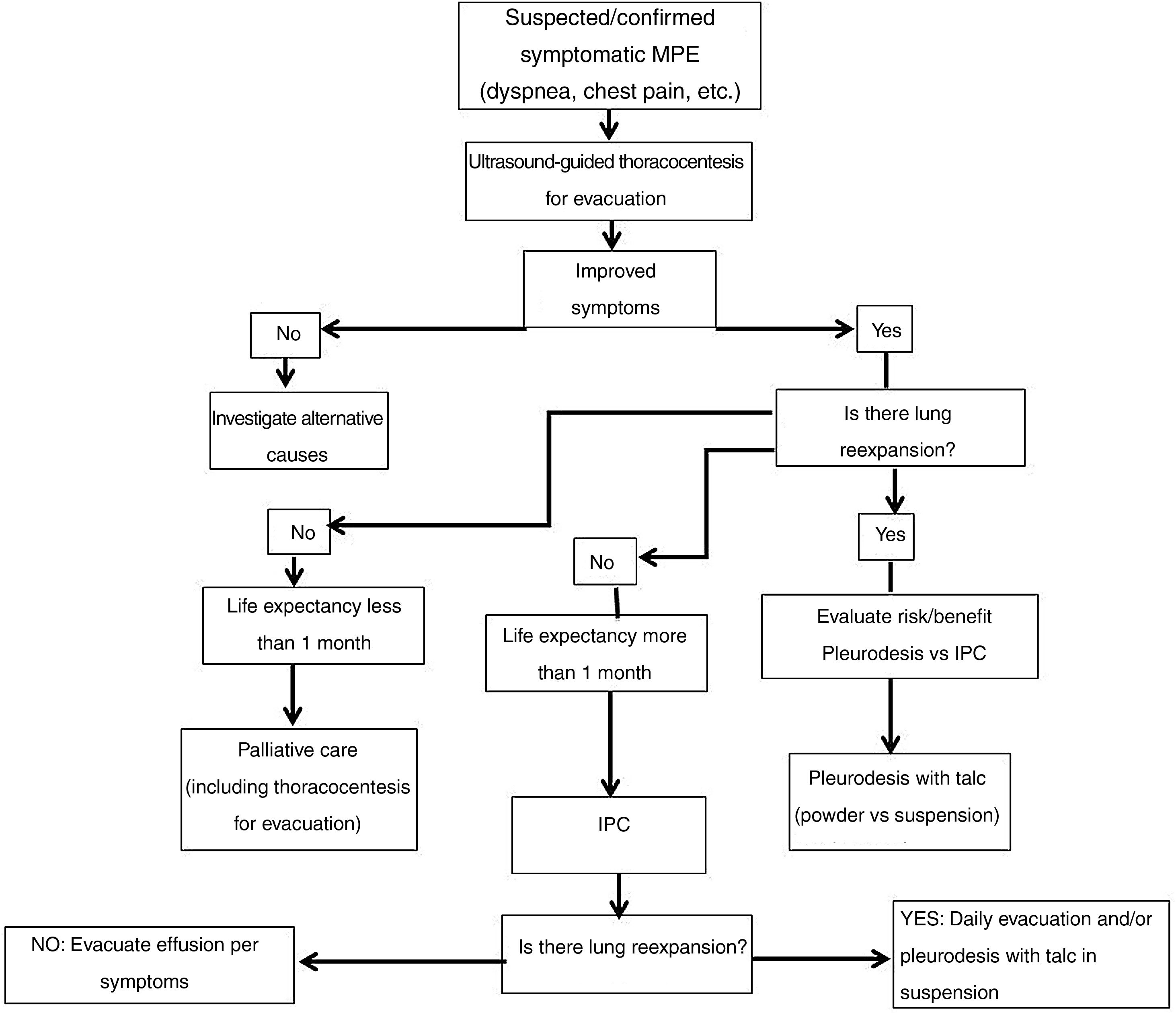
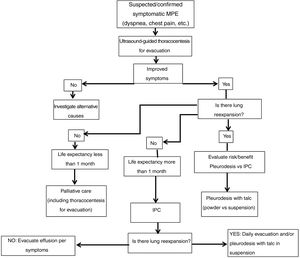
Fig. 4 shows the diagnostic algorithm for symptomatic MPE.
Promising studies are underway combining different techniques for the management of MPE (thoracoscopy/indwelling pleural catheter/talc pleurodesis).83
ConclusionsPD is a common and rapidly evolving disease. The frequency and complexity of pleural disease and the development of pleural procedures such as image-guided PB, new indications for medical thoracoscopy, and IPC for managing MPE and BP prompted an update of the latest recommendations for diagnosis and treatment of PE. Key points include promoting outpatient treatment, establishing a rapid and accurate diagnosis, and avoiding unnecessary tests. These guidelines offer practitioners the opportunity to update their knowledge and improve the comprehensive, standardized, quality care for PE patients.
FundingThese guidelines were developed and prepared without any external funding.
Conflict of interestsThe authors state that they have no conflict of interests.