Randomized controlled trials (RCT) have not demonstrated a role for continuous positive airway pressure (CPAP) on the secondary prevention of major cardiovascular events in obstructive sleep apnea (OSA) patients. However, participants in RCTs are substantially different from real-world patients. Therefore, we aimed to assess the effect of CPAP treatment on major cardiovascular events in real-world OSA patients.
MethodsPopulation-based longitudinal observational study including all OSA patients with an active CPAP prescription at the beginning of 2011 in Catalonia, Spain, that terminated CPAP treatment during 2011 and did not have CPAP prescriptions between 2012-2015; and propensity-score-matched OSA patients that continued CPAP treatment until the end of 2015 or death. Adjusted hazard ratios were used to assess the association between CPAP treatment and overall and cardiovascular mortality, cardiovascular hospitalizations, or major adverse cardiovascular events (MACEs).
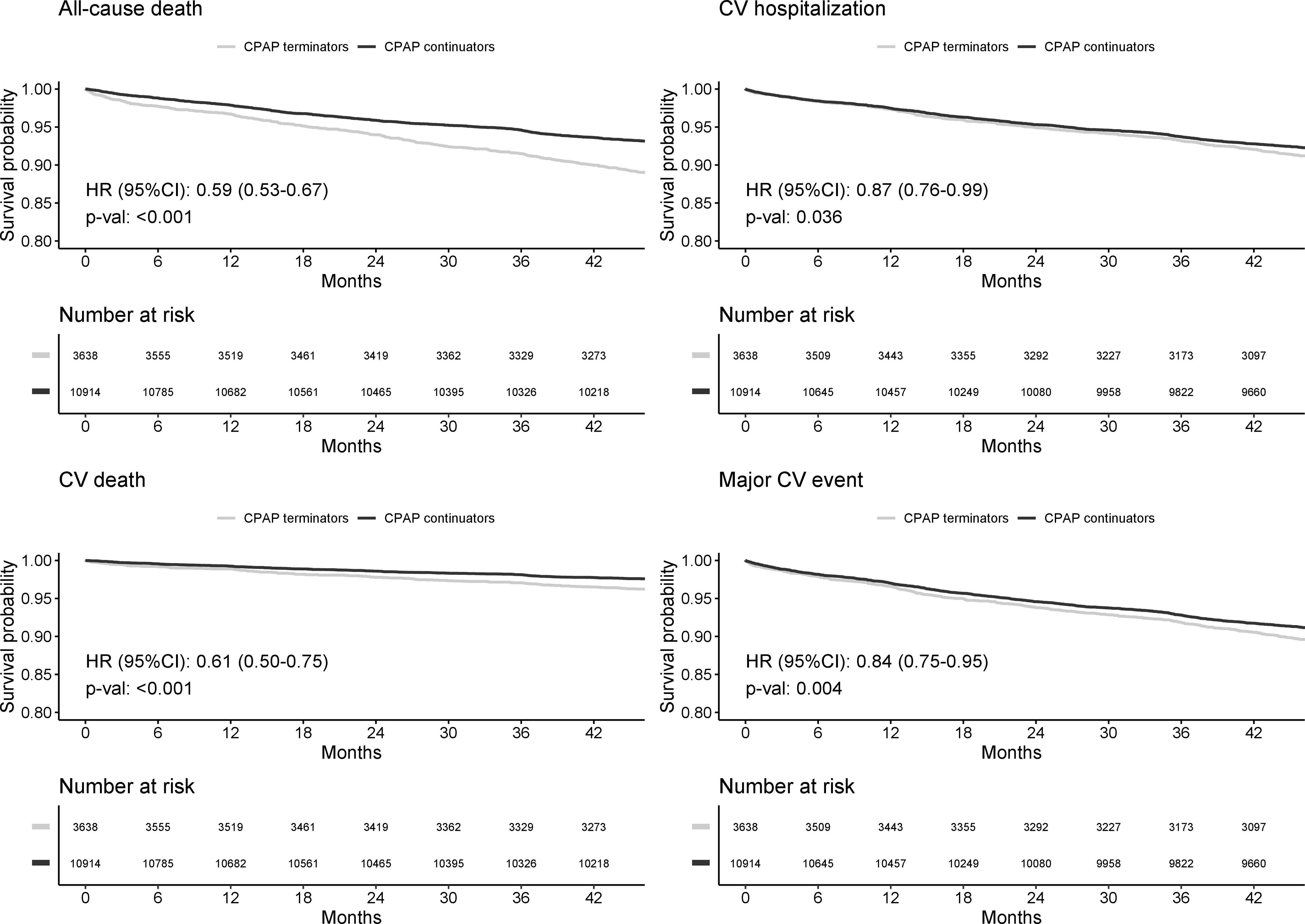
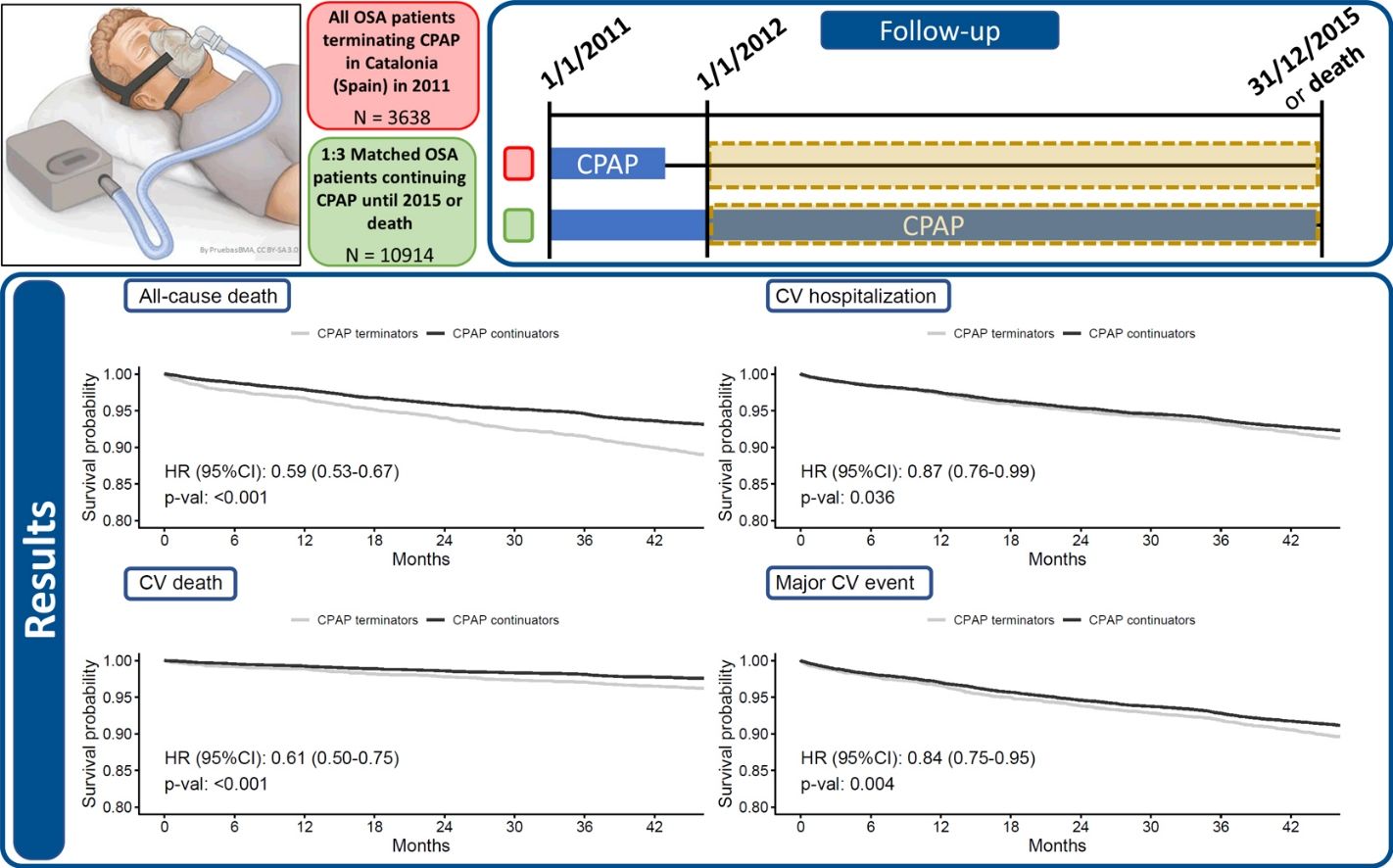
Results3638 CPAP terminators and 10,914 propensity-score-matched continuators were included (median age 67 [57–77] years, 71.4% male). During a median follow-up of 47.9 months CPAP continuators showed a lower risk of cardiovascular death than terminators (hazard ratio [HR]: 0.61; 95% confidence interval [CI]: 0.50–0.75) after adjusting by age, sex and key comorbidities. Similar results were found for cardiovascular hospitalizations (HR: 0.87; 95% CI: 0.76–0.99) and MACEs (HR: 0.84; 95% CI: 0.75–0.95).
ConclusionCPAP treatment continuation could be associated with a significantly lower risk of major cardiovascular events in real-world OSA patients. This result highlights the importance of including real-world patients in studies on OSA.
Obstructive sleep apnea (OSA) affects 20-30% of the adult population.1 OSA is characterized by upper airway collapse during sleep and encompasses increased morbidity and mortality due to its association with cancer, hypertension, and cardiovascular (CV), cerebrovascular, and metabolic diseases2; with increased inflammation, oxidative stress, sympathetic activation and hypercoagulability being the main involved mechanisms. Nocturnal continuous positive airway pressure (CPAP) is the standard treatment for OSA.3 CPAP improves daytime sleepiness and quality of life. It also improves several OSA severity markers like the apnea-hypopnea index (AHI), and moderately decreases arterial blood pressure in patients with resistant hypertension.3,4
CPAP treatment has long been suggested to have a role in the secondary prevention of CV complications.5 However, key randomized controlled trials (RCTs) did not support a role for CPAP in the secondary prevention of CV events in OSA patients with previous cerebrovascular and coronary heart disease,6 previous coronary heart disease,7 or previous acute coronary syndrome.8 Nevertheless, it should be noted that RCTs may face difficulties in replicating real-life scenarios. Due to the strict criteria for inclusion and exclusion, as well as ethical considerations, such as the need to randomize patients to receive or not receive CPAP treatment, the participants in RCTs may not accurately represent the entire population of individuals who use CPAP.9 For instance, a recent meta-analysis on the three major RCTs on the topic included an on-treatment analysis using marginal structural models showing a reduced risk of major adverse cardiac and cerebrovascular events associated with good adherence to CPAP (hazard ratio, 0.69 [95% CI, 0.52-0.92]),10 but the overall adherence to CPAP in the RCTs was very low (3.1h/night) as compared with data from subjects in research studies in the literature (4.5h/night)11 or real-world patients attended in US Centers of Medicare & Medicaid Services (4.7h/night).12 As a result, the generalizability of RCT results may be affected, and options such as observational designs using propensity scores in real-world patients have been proposed.13 In this sense, studies using population-based data have shown an association of CPAP treatment with lower overall mortality rates,14,15 but they did not tackle the topic of cardiovascular outcomes. In contrast, a recent large observational study including OSA patients prescribed with PAP showed a dose-response relationship between PAP adherence and the incidence of major CV events (MACEs).16 However, this study exclusively focused on PAP users, thereby excluding non-utilizers of PAP, and while effectively investigating whether a high adherence level to treatment yields superior outcomes compared to low adherence, it remained inconclusive regarding scenarios where PAP treatment is entirely absent. Therefore, a large population-based study on OSA patients, including CPAP users and non-users, tackling cardiovascular outcomes, is still missing.
Considering the conflicting previous evidence, we aimed to assess the effect of long-term CPAP treatment on major CV events in real-world OSA patients in a population-based longitudinal observational study. This approach benefited from the additional external validity of population-based studies at the cost of the limited data availability of real-world data.
MethodsStudy Design and PopulationThis was a population-based longitudinal observational study including all OSA patients older than 30 with an active CPAP prescription at the beginning of 2011 in Catalonia, Spain, that terminated CPAP treatment during 2011 and did not have any other CPAP prescriptions between 2012-2015 (CPAP terminators); and similar propensity-score-matched OSA patients that continued CPAP treatment until Dec 31st 2015 or their death (CPAP continuators). The follow-up time included the period between Jan 1st 2012 and Dec 31st 2015. The present analysis did not include patients who received CPAP treatment through private health services.
Propensity Score MatchingCPAP terminators were matched 1:3 with CPAP continuators using the nearest neighbour propensity score matching method based on age, sex, and baseline comorbidities including chronic obstructive pulmonary disease (COPD), diabetes, chronic heart failure, ischemic heart disease, high blood pressure, and chronic renal failure. Propensity scores were estimated using a generalized linear model.
Variables and Data CollectionThe current study is fully based on registry data provided by the Agency for Healthcare Quality and Evaluation of Catalonia (AQuAS - PADRIS program), which is a public entity attached to the Department of Health of the Government of Catalonia. CPAP prescriptions were identified based on invoiced services. Baseline information included age, gender, health region, date of CPAP prescription, and comorbidities (ICD-9). Outcome variables included occurrence and date of all-cause death and CV death (as coded using ICD-10 in the Catalan Statistical Bulletin of Death), CV hospitalizations, and major adverse cardiovascular events (MACEs) defined as a composite of CV death and CV hospitalization.
Statistical AnalysisParticipants’ baseline characteristics are described by mean (SD) or number (%). Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals for the association between CPAP treatment group and all-cause mortality, CV mortality, CV hospitalization, or major CV events. Models were adjusted by age, sex and baseline comorbidities (diabetes, chronic heart failure, COPD, high blood pressure, chronic renal failure, and ischemic heart disease). Kaplan-Meier curves were plotted to show the survival probability for the different outcomes through the follow-up. Stratified analysis according to sex, age (<70 or ≥70 years), baseline high blood pressure, and baseline chronic heart failure or ischemic heart disease were performed. Models including an interaction between CPAP treatment and sex were used to assess potential sex differences in the association between CPAP treatment and the outcomes. Similar models were done for older age (<70 or ≥70 years), baseline high blood pressure, and baseline chronic heart failure or ischemic heart disease. No models could be done for younger age (<40 or ≥40 years) due to the low number of events. The proportional hazards assumption was satisfied in all models. The statistical level of significance was fixed at 0.05. All analyses were performed using R statistical software, version 4.2.2.
Ethics and Data ProtectionThe ethics committee of University Hospital Arnau de Vilanova from Lleida (Catalonia) approved this study (2015, CEIC-1430). Patient informed consent was not required since all data were anonymized. All data was handled in agreement with national and international law.
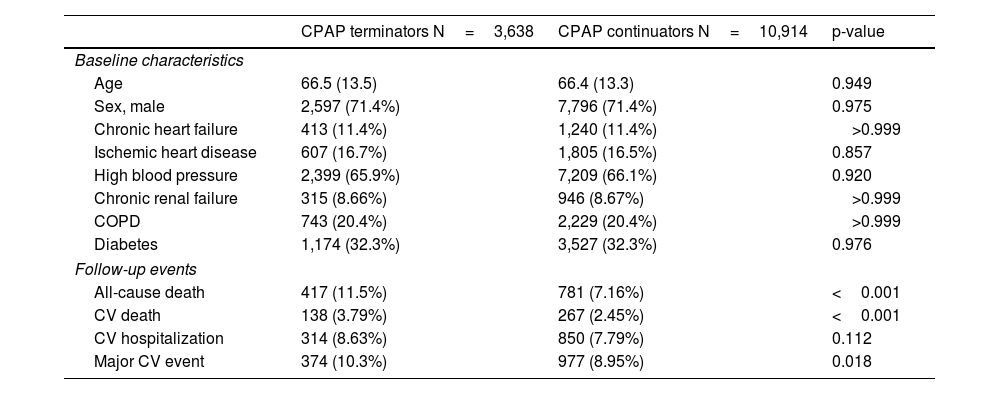
ResultsUp to 3,638 CPAP terminators and 44,996 CPAP continuators were available in the 2011 Catalan registries provided by AQuAS. Table E1, in the online data supplement, summarizes the propensity score matching process, which included 48,634 observations. Finally, 3,638 CPAP terminators and 10,914 propensity-score-matched continuators were included in the current analyses. Included subjects had a median age 67 (57–77) years and 71.4% were male. The characteristics of the included patients are shown in Table 1, which also includes the outcome events recorded during the median 47.9 months of follow-up. Statistically significant differences were detected for all-cause death, CV death, and MACEs, but not for CV hospitalizations (p=0.112).
Patients’ baseline characteristics and events during the follow-up according to CPAP termination or continuation.
| CPAP terminators N=3,638 | CPAP continuators N=10,914 | p-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age | 66.5 (13.5) | 66.4 (13.3) | 0.949 |
| Sex, male | 2,597 (71.4%) | 7,796 (71.4%) | 0.975 |
| Chronic heart failure | 413 (11.4%) | 1,240 (11.4%) | >0.999 |
| Ischemic heart disease | 607 (16.7%) | 1,805 (16.5%) | 0.857 |
| High blood pressure | 2,399 (65.9%) | 7,209 (66.1%) | 0.920 |
| Chronic renal failure | 315 (8.66%) | 946 (8.67%) | >0.999 |
| COPD | 743 (20.4%) | 2,229 (20.4%) | >0.999 |
| Diabetes | 1,174 (32.3%) | 3,527 (32.3%) | 0.976 |
| Follow-up events | |||
| All-cause death | 417 (11.5%) | 781 (7.16%) | <0.001 |
| CV death | 138 (3.79%) | 267 (2.45%) | <0.001 |
| CV hospitalization | 314 (8.63%) | 850 (7.79%) | 0.112 |
| Major CV event | 374 (10.3%) | 977 (8.95%) | 0.018 |
Mean (SD) or n (%). Follow-up from Jan 1st 2012 to Dec 31st 2015.
CPAP: continuous positive airway pressure; COPD: chronic obstructive pulmonary disease; CV: cardiovascular.
Figure 1 shows the Kaplan-Meier curves and adjusted HR 95% CI, of the survival probability for all-cause death, CV death, CV hospitalization and MACEs through the follow-up, adjusted by age, sex and baseline comorbidities (diabetes, chronic heart failure, COPD, high blood pressure, chronic renal failure, and ischemic heart disease). All scenarios showed a statistically significant protective effect of CPAP on the outcomes: all-cause death (hazard ratio [HR]: 0.59; 95% CI: 0.53–0.67), CV death (HR: 0.61; 95% CI: 0.50–0.75); CV hospitalization (HR: 0.87; 95% CI: 0.76–0.99) and MACEs (HR: 0.84; 95% CI: 0.75–0.95).
Kaplan-Meier curves and adjusted Hazard Ratios (HR) 95% CI, of the survival probability for All-cause death, Cardiovascular (CV) death, CV hospitalization and Major CV event through the follow-up. All models are adjusted for age, sex and baseline comorbidities (diabetes, chronic heart failure, COPD, high blood pressure, chronic renal failure, and ischemic heart disease). Number of subjects at risk at each time point are reported under each plot.
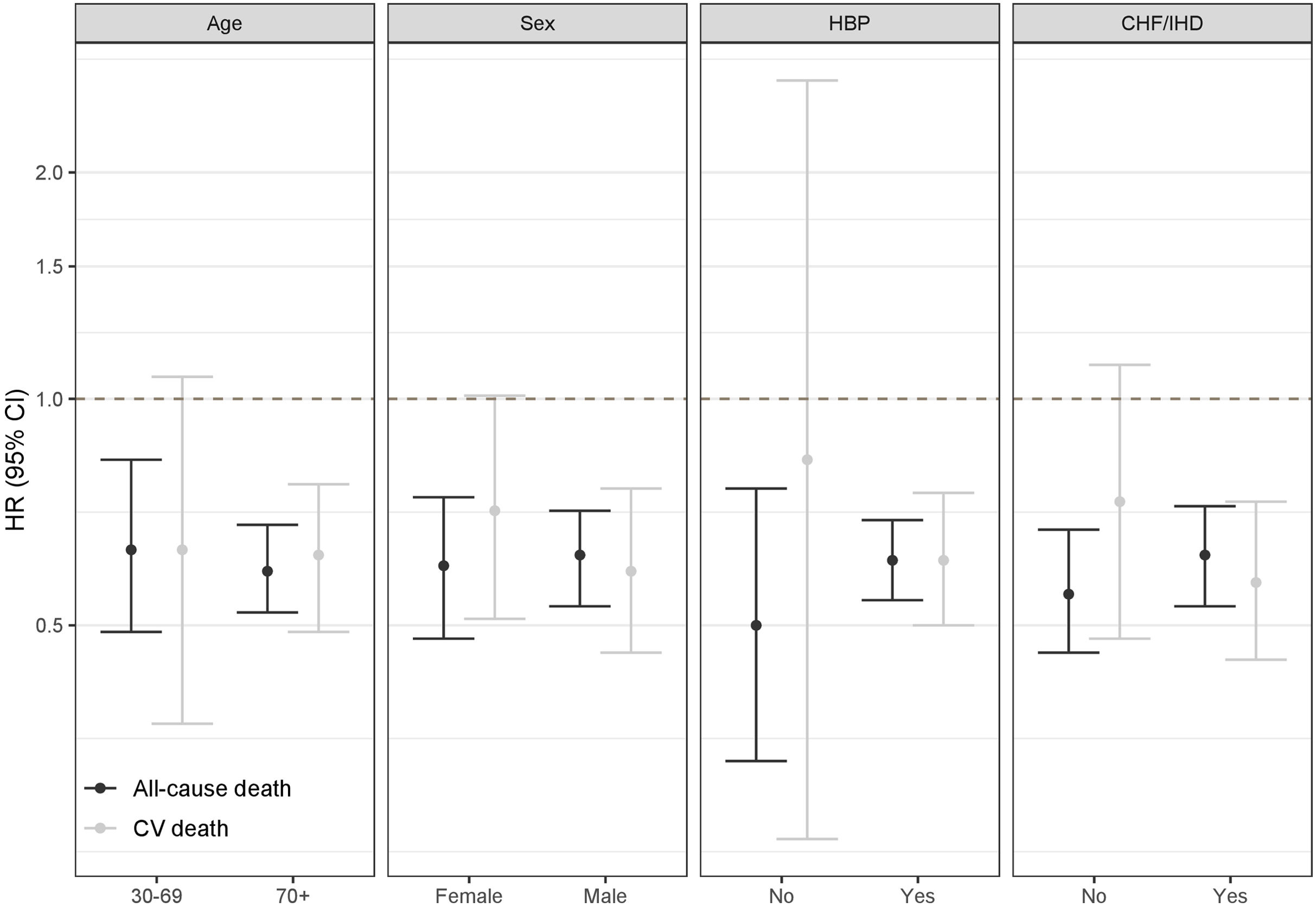
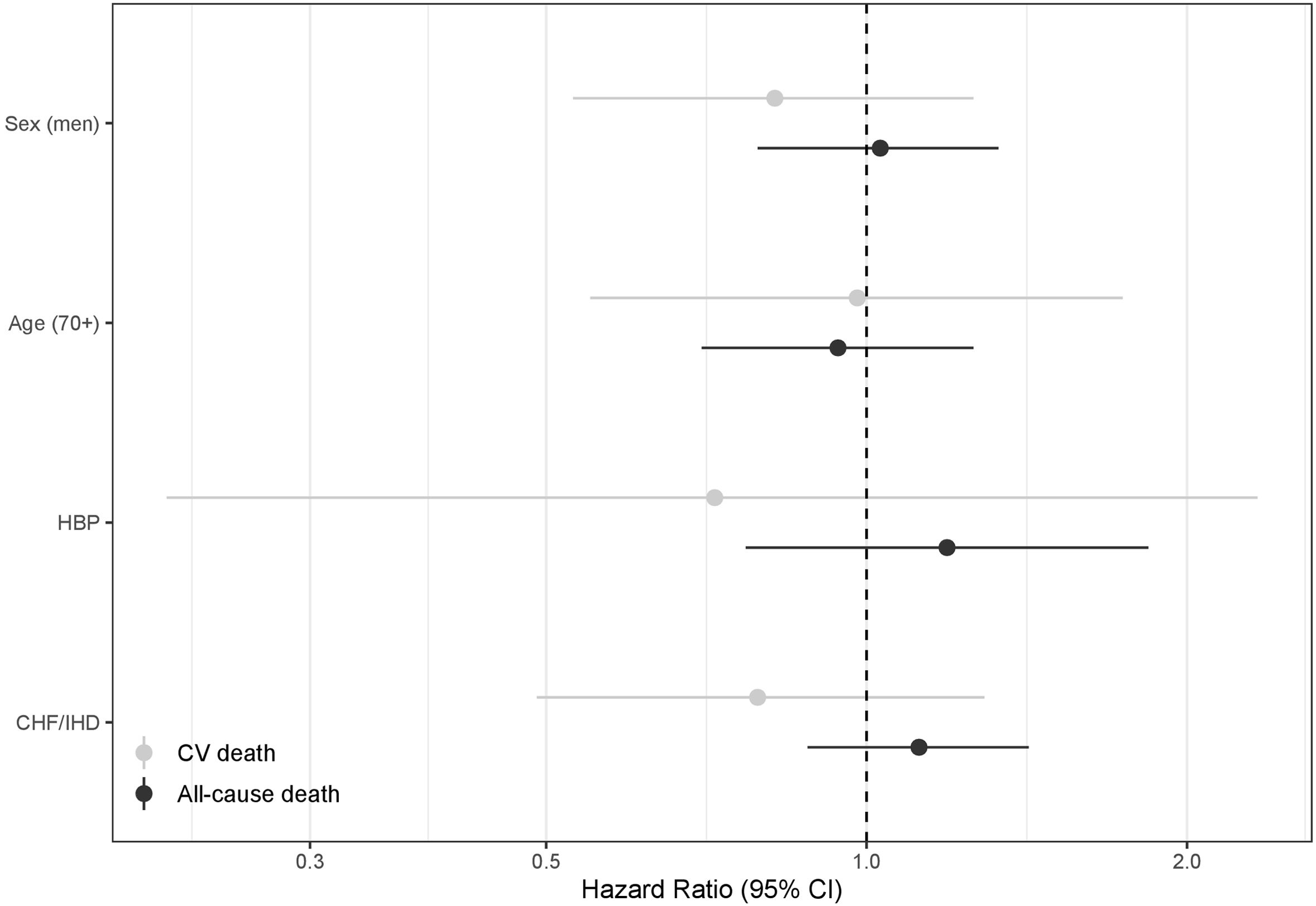
Stratified models for all-cause death and CV death according to age (<70 or ≥70 years), sex, baseline high blood pressure (HBP), and baseline chronic heart failure or ischemic heart disease (CHF/IHD) are shown in Figure 2. Although no statistically significant differences were found in any of the scenarios, a higher protective effect of CPAP on all-cause death was suggested among patients without baseline HBP; and lower protective effect of CPAP on CV death was suggested among patients without baseline CHF/IHD. These results remained non-statistically-significant in models including interaction terms (figure 3).
Hazard ratios of stratified models for the effect of CPAP treatment on cardiovascular (CV) and all-cause death according to age (<70 or ≥70 years), sex, baseline high blood pressure (HBP), and baseline chronic heart failure or ischemic heart disease (CHF/IHD). Models for age and sex are crude. Models for HBP and CHF/IHD are adjusted for age and sex.
Hazard ratios of the interaction terms for CPAP treatment and sex (men), age (≥70), baseline high blood pressure (HBP), and baseline chronic heart failure or ischemic heart disease (CHF/IHD); in relation to cardiovascular (CV) and all-cause death. Models for sex and age are crude. Models for HBP and CHF/IHD are adjusted for age and sex. Hazard ratios for the interaction terms with 95% confidence intervals crossing the dashed vertical line indicate that the association between CPAP treatment and CV/all-cause death was not statistically different in the two categories of the stratified variable (i.e. age <70 and ≥70 years).
The longitudinal analysis of all OSA patients terminating CPAP treatment in Catalonia in 2011 and similar propensity-score-matched CPAP continuators showed a significant protective effect of CPAP on all-cause death, CV death, CV hospitalizations, and major CV events during the period 2012-2015. No statistically significant differences were found when stratifying by age, sex, baseline HBP, and baseline CHF/IHD.
On the one hand, the current results are in contrast with the primary results of the main randomized controlled trials assessing the usefulness of CPAP for the secondary prevention of CV events in OSA patients, which reported null results.6–8 Furthermore, a meta-analysis of RCTs on this issue by Labarca et al. confirmed the null results, and hinted that issues with study design and implementation, including potential biases, variations in CPAP adherence, and study population characteristics, may have weakened the findings.17 Only when the results have been analysed in terms of having a good treatment adherence (thus resembling the majority of CPAP users in Catalonia), CPAP treatment has been associated with reduced MACE recurrence.10 It is key to notice that patients enrolled in RCTs may not be comparable to real-world patients,9 mostly due to tight inclusion and exclusion criteria and ethical considerations on the need to randomize patients to not receiving CPAP treatment. Therefore, the generalizability of RCT results may be limited, and alternative approaches such as observational designs using propensity scores in real-world patients, like in the current analyses, could provide results with a higher external validity.13 In contrast, RCTs do avoid healthy adherer and healthy user biases (as long as being analysed as intention to treat18), which may affect other study designs.19
On the other hand, the results are well-aligned with those of real-world data studies involving large populations of CPAP-treated OSA patients. In this sense, the relationship between CPAP termination and all-cause mortality has already been demonstrated using data from a French nationwide database,15 with CPAP continuation being associated with reduced all-cause mortality (HR: 0.61; 95% CI: 0.57–0.65), exactly in the same magnitude as reported in the current analyses (HR: 0.59; 95% CI: 0.53–0.67). Similarly, results of a study on the effect of long-term CPAP use on mortality and morbidity showed that it could reduce mortality and incidence of type II diabetes mellitus and CV disease.20 Although no similar approach has tackled the association for CV death, CV hospitalizations or major CV events, several real-world data studies have assessed whether CPAP adherence could reduce CV events. In 2022, Bailey et al. reported that CPAP adherence could reduce the risk of 30-day hospitalization readmission of hospitalized patients with OSA and CVD.21 Similarly, real-life clinical data from the Pays de la Loire Cohort, including more than five-thousand OSA patients, showed that high PAP treatment adherence (6–7h/night) and very high adherence (>7h/night) were associated with lower incidence of major adverse CV events when compared to non-adherent patients (0–4h/night), HR: 0.75; 95% CI: 0.62–0.92, and HR: 0.78; 95% CI: 0.65–0.93, respectively.16
The current results did not show significant differences in the relationship between CPAP and the studied outcomes according to age, sex, baseline HBP, and baseline CHF/IHD. Results for HBP and CHF/IHD imply that CPAP could have a role in both the primary and secondary prevention of MACEs although we could only analyse overall and CV mortality. Other real-world data analyses have reported differences according to sex,16,22 or previous CV disease,16 and could hint the existence of different OSA phenotypes which are not easily established in large registry-based real-world data studies. Indeed, results from Hong Kong support a potential role for OSA phenotypes on the association between CPAP treatment and CV outcomes.23 In this sense, it would be desirable to enrich current population-based registries with further variables that could allow for better patient characterization. Overall, it seems plausible that CPAP could potentially act in a primary prevention scenario, where it could revert some underlying mechanisms when they are still reversible or partially reversible; and, in secondary prevention scenarios, but most likely only in certain profiles of patients such as those reported in the Hong Kong cohort.23
The link between OSA and CV disease has been widely recognized.2,24,25 Multiple studies have identified CPAP treatment as effective in relation to different aspects of CV disease such as blood pressure,26,27 echocardiographic parameters,28 left ventricular function,29 or venous thromboembolisms,30 among others. Therefore, the current and previous results from real world data analyses have a solid research evidence backing their plausibility.
The current results confirm that overall, at the population level, the continued use of CPAP is associated with reduced CVD outcomes. However, it cannot be inferred that all OSA patients would benefit of an added CV protection by regularly using CPAP. Large RCTs have shown that their targeted participants do not benefit from CPAP treatment in terms of CV outcomes. Although we do know that only up to 20% of real-world OSA patients meet eligibility criteria for RCTs,9 this proportion should not be underestimated. Therefore, the need for adequate OSA patient characterization is as important as ever, especially when trying to understand which patients could benefit the most of the CV protection offered by adequately treating OSA.31–33
The current study's approach was intended to circumvent the restrictions imposed by randomized controlled settings, such as stringent inclusion and exclusion criteria, or ethical considerations surrounding the withholding of CPAP treatment from patients in the control group. The use of a large population-based public dataset had two main advantages. First, it ensured an accurate picture of CPAP-treated OSA patients in Catalonia, as all patients prescribed with CPAP therapy through the Catalan Health System in 2011 were considered for the propensity-score matching, thus enhancing external validity. Second, and given the median follow-up of 47.9 months, it allowed for a sufficient number of recorded events. In contrast, several drawbacks should be noticed. First, the limited number of available variables in the public dataset did not allow controlling for potentially important confounding factors such as OSA severity (either in terms of AHI or other indices), OSA symptoms (sleepiness), adherence to CPAP treatment, the level of systolic blood pressure, body mass index, tobacco exposure, alcohol, physical activity, and the baseline severity of each comorbidity. In fact, comorbidity assessment was limited to ICD-9 codes, which could be a source of bias on its own. Some of the abovementioned shortcomings in terms of available variables can be partially overcome by population-based information from Catalonia. For instance, official data provided by AQuAS states that the overall CPAP compliance in Catalonia is high (>80% of treated patients with>3hours/night), which is in line with historic data showing that 80% of patients have a compliance above 4h/night in the Health Region of Barcelona.34 Second, the results may be affected to a certain degree by healthy adherer or healthy user bias,19 which could not be tackled by the propensity score matching as the study database did not have information on adherence to other treatments that could be used as a surrogate. However, as previously discussed, the results from the Pays de la Loire Cohort, considering CV active drug adherence as a proxy of healthy adherer effect, were very similar to those reported here in both direction and magnitude of the associations.16 Third, the study database did not include subjects who were prescribed CPAP therapy from private medical centres. However, in Catalonia, nearly 90% of complex treatments, including CPAP therapy, are prescribed through the public health system. Fourth, no information on the motivation for CPAP termination was available. Potential biases related to this factor were mitigated by deciding to start the follow-up period in 2012, so CPAP termination was not motivated by a worsening of health status during the follow-up. Finally, the limited number of variables available for propensity score matching could not suffice to rule out potential residual confounding by known or unknown factors, such as socioeconomic status or level of health literacy and self-care. Overall, the current manuscript presents results with a high external validity, as this is the very first population-based study on this topic, but with a higher potential risk of biases. Therefore, the results should be interpreted cautiously and in the light of the rest of the literature.
In conclusion, the analysis of all OSA patients terminating CPAP treatment in Catalonia during 2011, and similar propensity-score-matched CPAP continuators, suggests that CPAP treatment continuation could be associated with a significantly lower risk of all-cause death, CV death, CV hospitalizations, and MACEs. The results of other real-world studies added to those reported here, which are especially valuable given their population-based nature, as well as those of RCTs when analysed in the light of good treatment adherence suggest that CPAP treatment continuation and adherence could lower the risk of CV events. Moreover, the combined results of RCTs and real-world studies suggests the existence of different OSA patient phenotypes in which CPAP treatment would be differently associated to CV outcomes. Therefore, OSA patient characterization either with new or conventional metrics is as important as ever, especially when trying to understand which patients could benefit the most of the CV protection offered by adequately treating OSA.
Funding InformationThis study was supported by Instituto de Salud Carlos III (ISCIII) through the project “PI18/00502”, co-funded by ERDF, “A way to make Europe”; Spanish Respiratory Society (SEPAR); Sociedad Española de Sueño (SES); Societat Catalana de Pneumologia (SOCAP) - Beca Esteve Teijin; Associació Lleidatana de Respiratori (ALLER); and ResMed Foundation. Jordi de Batlle acknowledges the Catalan Departament de Salut (PERIS 2016: SLT002/16/00364) and Instituto de Salud Carlos III (ISCIII; Miguel Servet 2019: CP19/00108), co-funded by the European Social Fund (ESF), “Investing in your future”. MS received financial support from a “Ramón y Cajal” grant (RYC2019-027831-I) from the “Ministerio de Ciencia e Innovación - Agencia Estatal de Investigación” cofunded by the European Social Fund (ESF)/“Investing in your future”. FB is supported by the ICREA program, Generalitat de Catalunya. The funders did not play any role in the study design, study analysis, manuscript preparation or decision to publish this work.
Author ContributionsEG-L had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JdB wrote the first draft of the manuscript. JdB, EG-L and FB defined the study design. All authors contributed to the interpretation of results and critically reviewed the manuscript.
Conflicts of interestAll authors state that they have no conflicts of interest to declare.
The authors acknowledge the Catalan Health Quality and Assessment Agency (AQuAS) and its Public Data Analytical Program for Health Research and Innovation (PADRIS Program) for kindly providing all required data at the request of the Master Plan for Respiratory Diseases (Ministry of Health, Catalonia).