A bronchopleural fisutal is a comunication between the pleural space and the bronchial tree. The most common cause of BPF is dehiscence of the surgical suture after lung resection, which happens between in about 0.8% and 15% of cases. Therapeutic options are limited and depend, among other factors, on the size and site of the fistula. Endoscopic treatment is a feasible therapeutic option, particularly in BFP measuring <5mm.1 Good final outcomes of BPF closure with Histoacryl® (N-butyl-2-cyanoacrylate) in respiratory endoscopy have been reported.2 However, in practice, the rapid polymerization of N-butyl-2-cyanoacrylate makes management and endoscopic placement difficult. Other cyanoacrylates that polymerize at a different rate and at different temperatures are available, and these may be easier to use with an endoscope. No comparative studies have been published on the use of other cyanoacrylates, such as Glubran® 2 (N-butyl-2-cyanoacrylate+metacryloxisulfolane), as synthetic surgical sealants.
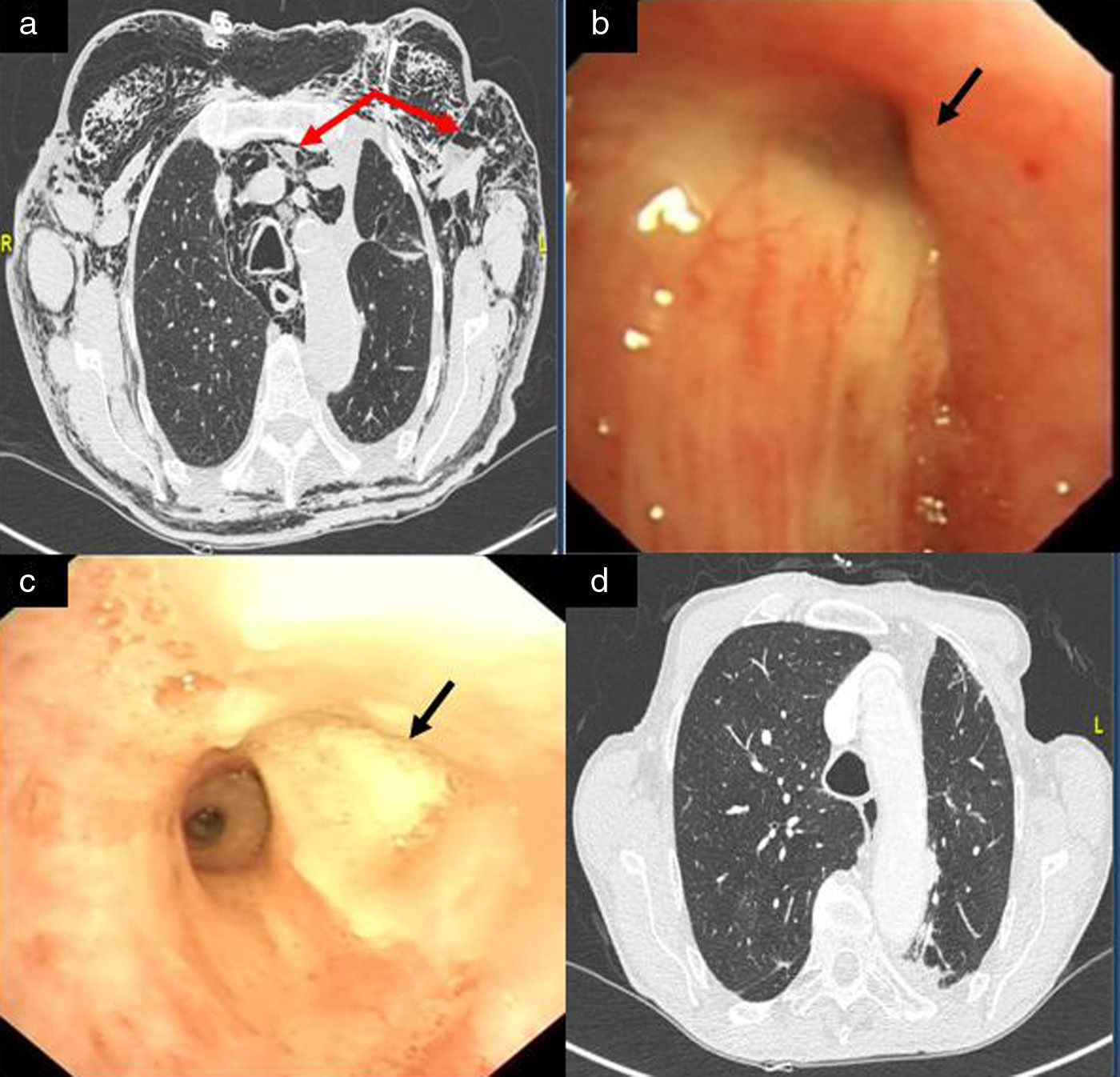
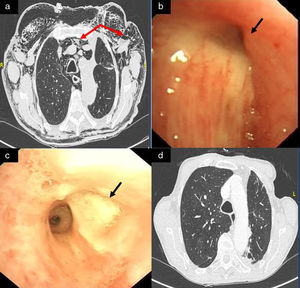
We report the case of an 80-year-old man, active smoker, COPD, GOLD B, who underwent left posterolateral thoracotomy with lobectomy of the left upper lobe (LUL) and systemic lymph node resection, with a diagnosis of squamous lung cancer, stage pT2aN0M0. Two weeks after the intervention, the patient presented progressive subcutaneous and mediastinal emphysema, with significant facial edema, but no upper airway compromise (Fig. 1a). During the endoscopic examination with flexible bronchoscope, a 3mm fistula was observed in the LUL lobectomy stump wound (Fig. 1b). In view of the endoscopic findings, persistent air leak, and worsening subcutaneous emphysema, the fistula was sealed using cyanoacrylate, as follows: 1ml N-butyl-2-cyanoacrylate+metacryloxisulfolane (Glubran® 2 Ref.G-NB-2, GEM srl, Italy), applied using a syringe via a 5 Fr angioplasty catheter (Angiographic Cathether Tempo® Vertebral [VERT] Ref. 451-514H0 Cordis®). Due to the characteristics of the sealant, a safety margin between the distal tip of the catheter and the bronchoscope lens was maintained. Immediately after applying the bronchial sealant, complete closure of the fistula was confirmed (Fig. 1c), the bronchoscope was withdrawn together with the angioplasty catheter placed in the working channel to avoid any sealant remnants entering the interior of the working channel and damaging the equipment during withdrawal. The distal end of the catheter was then closed to avoid any sealant remnant entering the working channel. Five days after the application of Glubran® 2, correct closure of the fistula was confirmed in a second endoscopic procedure; the patient had improved clinically with progressive resolution of the subcutaneous emphysema. The patient was followed up as an outpatient, and a follow-up chest computed tomography was performed 6 months after surgery, confirming clinical stability and complete resolution of the subcutaneous emphysema (Fig. 1d), with no recurrence or other serious long-term complications.
(a) Significant mediastinal and subcutaneous emphysema; (b) LUL lobectomy surgical stump with a 3mm bronchial fistula in the upper area; (c) bronchopleural fistula closure after instillation of Glubran® 2; and (d) resolution of mediastinal and subcutaneous emphysema on follow-up chest CT, 6 months after instillation of the bronchial sealant.
The physical and chemical properties of cyanoacrylate derivatives were described in 1959 by Coover et al.3 Methyl-2-cyanoacrylate was the first derivative used as surgical glue. Differences in polymerization speed depend on chain length: short-chain cyanoacrylates are faster, but they also cause greater cytotoxicity and inflammatory reactions. Currently, long-chain cyanoacrylates (butyl and octyl-cyanoacrylate) are more commonly used as surgical sealants in different medical and surgical areas.
The use of endobronchial valves4 and other biological materials, such as stromal cell preparations,5 have been described as possible treatments for bronchopleural fistula. With regard to sealants, many materials and devices offering similar sealing results have been used, although no comparative studies have been published on the use of different products. Glubran® 2 is a new cyanoacrylate derivative that demonstrates excellent capacity for joining biological material. The polymerized layer is highly resistant to rupture, and polymerization time is slow. In our patient, BPF was closed with the use of cyanoacrylate and the air leak was controlled in a single endoscopy procedure, placement of the material was easy, and there were no complications. In our experience, the use of Glubran® 2 for endoscopic sealing of BPF was a feasible, safe procedure, offering immediate closure with no subsequent complications.
Please cite this article as: Cepeda S, Pajares V, Trujillo-Reyes JC, Torrego A. Utilización de un nuevo cianoacrilato como sellante bronquial en el tratamiento endoscópico de la fístula broncopleural. Arch Bronconeumol. 2017;53:168–169.