Obstructive sleep apnea (OSA) increases the risk of type 2 diabetes, and hyperinsulinemia. Pregnancy increases the risk of OSA; however, the relationship between OSA and gestational diabetes mellitus (GDM) is unclear. We aimed (1) to evaluate OSA prevalence in GDM patients; (2) to assess the association between OSA and GDM; and (3) to determine the relationships between sleep parameters with insulin resistance (IR).
MethodsA total of 177 consecutive women (89 with GDM, 88 controls) in the third trimester of pregnancy underwent a hospital polysomnography. OSA was defined when the apnea-hypopnea index (AHI) was ≥5h−1.
ResultsPatients with GDM had higher pregestational body mass index (BMI) and neck circumference than controls, but no differences in snoring or OSA-symptoms, or AHI (3.2±6.0 vs. 1.9±2.7h−1, p=.069). OSA prevalence was not significantly different in both groups. We did not identify OSA as a GDM risk factor in the crude analysis 1.65 (95%CI: 0.73–3.77; p=.232). Multiple regression showed that total sleep time (TST), TST spent with oxygen saturation<90% (T90), and maximum duration of respiratory events as independent factors related with homeostasis model assessment of IR, while T90 was the only independent determinant of quantitative insulin sensitivity check index.
ConclusionOSA prevalence during the third trimester of pregnancy was not significantly different in patients with GDM than without GDM, and no associations between OSA and GDM determinants were found. We identified T90 and obstructive respiratory events length positive-related to IR, while TST showed an inverse relationship with IR in pregnant women.
La apnea obstructiva del sueño (AOS) aumenta el riesgo de diabetes tipo 2 e hiperinsulinemia. El embarazo aumenta el riesgo de AOS; sin embargo, la relación entre la AOS y la diabetes mellitus gestacional (DMG) no está clara. Nuestros objetivos fueron (1) evaluar la prevalencia de AOS en pacientes con DMG; (2) evaluar la asociación entre la AOS y la DMG; y (3) determinar las relaciones entre los parámetros del sueño y la resistencia a la insulina (RI).
MétodosUn total de 177 mujeres seleccionadas consecutivamente (89 de ellas con DMG, 88 controles) en el tercer trimestre del embarazo se sometieron a una polisomnografía hospitalaria. Se clasificó como AOS un índice de apnea-hipopnea (IAH) de ≥5h-1.
ResultadosLas pacientes con DMG presentaban un índice de masa corporal (IMC) pregestacional y una circunferencia del cuello más altos que los controles, pero no hubo diferencias en los ronquidos, otros síntomas de AOS o el IAH (3,2±6,0 frente a 1,9±2,7h-1, p=0,069). La diferencia en la prevalencia de AOS entre ambos grupos no fue significativa. No identificamos la AOS como un factor de riesgo de DMG en el análisis bruto (1,65; IC del 95%: 0,73-3,77; p=0,232). Mediante regresión múltiple se determinó que el tiempo total de sueño (TST), el TST pasado con una saturación de oxígeno <90% (T90) y la duración máxima de los eventos respiratorios eran factores independientes relacionados con el Homeostasis Model Assessment of IR (HOMA-IR), mientras que el T90 fue el único determinante independiente del Quantitative Insulin Sensitivity Check Index (QUICKI).
ConclusiónLa diferencia de prevalencia de AOS durante el tercer trimestre del embarazo no fue significativa entre las pacientes con DMG y aquellas sin DMG, y no se encontraron asociaciones entre los factores asociados a AOS y DMG. Identificamos que la T90 y la duración de los eventos respiratorios obstructivos estaban relacionados positivamente con la RI, mientras que el TST mostró una relación inversa con la RI en las mujeres embarazadas.
Gestational diabetes mellitus (GDM), defined as glucose intolerance that is first detected during pregnancy, is a major public health burden with prevalence ranging from 1% to 14%. Moreover, it is a well-established risk factor for adverse maternal and infant health outcomes, including preeclampsia, cesarean delivery, fetal macrosomia, and fetal death, as well as long-term risk of obesity, metabolic syndrome, and diabetes mellitus in both mother and offspring. Numerous studies have identified risk factors for GDM, such as advance maternal age, previous GDM, member of some ethnic groups, and obesity.1–4
Obstructive sleep apnea (OSA) is a common disorder characterized by the presence of repetitive episodes of total or partial airflow interruption in the upper airway during sleep.5 Prevalence of OSA during pregnancy increases due to some physiological changes, such as weight gain, hormonal changes, or modifications in the upper airway.6–8
Previous studies suggest a possible association between OSA and adverse pregnancy and fetal outcomes, such as GDM, preeclampsia, preterm birth, and neonatal low weight.9–11 Some risk factors for OSA, including obesity and increasing age, are shared by GDM. In addition, there is growing evidence that OSA is an independent risk factor for type 2 diabetes, and it has an adverse impact on glycemic control.12
There are several previous studies that evaluated the association between GDM and OSA, but most of them relied on self-related OSA symptoms,13 were retrospective,14,15 or did not measure electroencephalogram signals (EES).9,16 Moreover, reported findings have been inconsistent. Poor sleep quality as well as disturbed sleep at some time points are extremely common during pregnancy,17 but very few studies with polysomnography (PSG), the gold standard for the diagnosis of OSA during pregnancy,7 have been published, and they showed contradictory results.18–21 Furthermore, most of them were limited because they included small samples with high-risk pregnant women, mostly with obesity. Methodological differences, in terms of sleep assessment procedures, and study population may have contributed to the inconsistent findings of the relationship between OSA and GDM. Therefore more studies with attended PSG are clearly needed to clarify the influence of important confounding factors such as obesity, age, ethnicity, and comorbidities.
To address these limitations, we designed a case control study to compare the prevalence of OSA detected with PSG between patients with GDM and a control group of glucose-tolerant pregnant women. Moreover, we aimed to assess the potential association between OSA and GDM determinants. Lastly, we explored the relationships between sleep characteristics and carbohydrate metabolism.
MethodsStudy design and participantsWe performed a multicentre case-control study at three tertiary and university hospitals namely Son Espases; Miguel Servet; and Araba, all in Spain. We selected as cases consecutive singleton adult pregnant women in the third trimester (3T) with GDM. GDM diagnosis was made according to the fasting 3-h 100-g glucose tolerance testing.22 As controls, we randomly selected adult pregnant women in the 3T without GDM. Subjects were excluded if they fulfilled at least one of the following exclusion criteria: (1) unwillingness or inability to participate in the study; (2) previous OSA; (3) previous diagnosis of diabetes mellitus, pulmonary, heart, or kidney diseases; (4) complicated pregnancy; (5) 50g oral glucose administration >140 before 24th gestation week; (6) multiple gestation; (7) treatment with systemic corticosteroids; (8) imminent delivery due to maternal-fetal disease; and/or (9) any other concurrent severe medical condition that would, in the investigator's judgment, contraindicate patient participation in the study.
The STROBE standards for reporting observational studies were followed. The Institutional Ethic Committee of the Balearic Islands approved the study (IB1510/10PI) and all subjects gave their written informed consent.
Laboratory researchers and study personnel were blinded to maternal status.
Clinical and sleep evaluationAnthropometric, clinical, and sleep data, including Epworth Sleepiness Scale (ESS),23 were collected in all participants. Attended PSG was performed and manually scored using conventional criteria in each center.24,25 More detailed information is provided in supplemental material. Apnea-hypopnea index (AHI) was established as the number of apneas/hypopneas per hour of sleep. OSA was defined when AHI was ≥5h−1.26 Rapid eye movement (REM) AHI was calculated as the number of apneas/hypopneas during REM divided by total time in REM. AHI in supine was calculated as the number of apneas/hypopneas in supine divided by total sleep time (TST) in supine position. The mean SaO2 throughout the night, the minimum SaO2 (lowest values recorded during sleep), TST spent with SaO2<90% (T90), and the number of ≥3% drops in SaO2 per hour of sleep (desaturation index [DI]) were also computed.
Laboratory determinationsThe morning after PSG, blood samples were collected in fasting conditions. Homeostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated by the usual formulas,27,28 More detailed information is provided in supplemental material.
Statistical analysisBased on the previous description of an OSA prevalence of 15.4% in the third trimester of pregnancy,19 and accepting an alpha risk of 0.05 and a beta risk of 0.2 in a one-sided test without considering drop-outs during the crossover evaluation, 88 subjects were necessary in each group to detect as statistically significant a prevalence of at least twice higher in women with GDM.
Data are presented as mean±standard deviation or percentage. Differences between groups were analyzed using Student's t test for continuous variables and Fisher's exact test (two-tailed) or chi-squared test for categorical variables. To examine associations between groups and the variables, odds ratios (OR) and 95% confidence intervals (CI) in the univariate analyses were calculated. Correlations between variables were examined by Pearson's correlation. To identify independent relationships, those variables that reached statistical significance in the bivariate correlation analysis were then introduced in a stepwise multiple linear regression analysis together with anthropometric data (age, gestational weight gain, body mass index, neck circumference, and waist–hip ratio). Stepwise criteria were a probability of the F-distribution test to enter <0.05 and a probability of the F-distribution test to remove >0.10. The assumptions of linearity and distributional normality were controlled for all variables. Homoscedasticity was explored by scatter plots of the standardized residuals against standardized predicted values and by Levene's test for equality of variances. A two-sided p value less than 0.05 was considered significant. The statistical software used was SPSS v.26 (IBM).
ResultsStudy subjects’ characteristicsFig. 1 presents the CONSORT flow chart of study subjects. From a total of consecutive 274 pregnant women considered, finally 89 newly diagnosed patients with GDM and 88 control pregnant women were included. Included women were mainly Caucasian (n=157, 89%) and nulliparous (n=117, 67%), with a mean±SD age of 34±4 years, and 18 (10.2%) had obesity (body mass index [BMI]≥30kg/m2) before gestation. Among the GDM participants, 33 (37.1%) required treatment with insulin, 3 (3.4%) were treated only with oral hypoglycemic agents, and 53 (59.6%) were diet-controlled.
The main demographic, clinical, and functional characteristics are shown in Table 1. Patients with GDM had greater pre-gestational and gestational BMI; however, weight gain at 3T was higher among control women. Moreover, neck circumference was significantly higher in GDM patients compared with control pregnant women. The remaining anthropometric characteristics, smoking and alcohol drinking, physical examination, and blood pressure were similar in GDM and control groups.
Clinical and functional characteristics of the study subjects.
| Gestational diabetes mellitus (n=89) | Control pregnant women (n=88) | p value | |
|---|---|---|---|
| Age, yr | 35±4 | 34±4 | 0.10 |
| Gestational age, weeks | 34.5±3.0 | 33.6±3.1 | 0.059 |
| Caucasian ethnicity, n (%) | 78 (87.6) | 79 (90.8) | 0.326 |
| First pregnancy, n (%) | 47 (54.0) | 48 (54.5) | 0.694 |
| Pregestational body mass index, kg/m2 | 25.6±5 | 22.9±3.2 | 0.00 |
| Pregestational obesity, n (%) | 15 (16.9) | 3 (3.5) | 0.00 |
| Gestational weight gain, kg | 8.5±5 | 11.3±4.4 | 0.00 |
| Gestational body mass index, kg/m2 | 28.8±4.8 | 26.9±2.9 | 0.00 |
| Pregestational smokers, % | 27.3 | 22.7 | 0.215 |
| Pack/yr | 3.6±5.3 | 4.2±8.1 | 0.598 |
| Neck circumference, cm | 34.4±3 | 33.5±2.3 | 0.03 |
| Waist–hip ratio | 1±0.07 | 0.99±0.05 | 0.184 |
| Mallampati, n (%) | 0.416 | ||
| Class I | 23 (26.4) | 27 (31.4) | |
| Class II | 22 (25.3) | 28 (32.6) | |
| Class III | 17 (19.5) | 14 (16.3) | |
| Class IV | 25 (28.7) | 17 (19.8) | |
| Micrognatia, % | 2.4 | 5.8 | 0.227 |
| High arched palate, % | 5.9 | 1.2 | 0.103 |
| Enlarged uvula, % | 4.7 | 0 | 0.059 |
| Large soft palate; % | 3.5 | 1.2 | 0.306 |
| Tonsillar hypertrophy | 2.4 | 2.3 | 0.685 |
| Gestational smokers, % | 11.2 | 8 | 0.056 |
| Pregestational alcohol intake, g/day | 0.98±4 | 1.1±5 | 0.891 |
| Systolic BP, mmHg | 109±12 | 106±10 | 0.060 |
| Diastolic BP, mmHg | 67±9 | 65±8 | 0.192 |
| Glucose, mg/dL | 76±9 | 76±8 | 0.832 |
| Cholesterol, mg/dL | 258±52 | 273±50 | 0.051 |
| Triglycerides, mg/dL | 226±99 | 203±83 | 0.104 |
| HDL cholesterol, mg/dL | 67.5±22.8 | 76.2±33.8 | 0.047 |
| Triglycerides/HDL cholesterol | 3.7±2.3 | 2.9±1.3 | 0.006 |
| Urea, mg/dL | 21.1±6.4 | 17.8±4.7 | 0.000 |
| Aspartate aminotransferase, U/L | 21.5±13 | 17.8±7.9 | 0.042 |
| Hemoglobin A1c, % | 5.3±0.8 | 5.3±1.1 | 0.867 |
| Insulin, μUI/mLa | 12.1±7.0 | 11.9±5.1 | 0.817 |
| HOMA-IRa | 2.3±1.4 | 2.2±1.1 | 0.901 |
| QUICKIa | 0.35±0.04 | 0.34±0.02 | 0.334 |
| Cortisol, mg/dL | 23.2±6.1 | 25.0±7.2 | 0.089 |
| Fibrinogen, mg/dL | 640±101 | 592±76 | 0.001 |
| Hemoglobin, g/dL | 12.1±1.1 | 11.7±0.9 | 0.003 |
| Leucocytes, 103/uL | 8.5±2.1 | 9.4±2.1 | 0.006 |
| Platelets, 103/uL | 215±58 | 229±54 | 0.098 |
Values represent mean±SD or percentage. Abbreviations: Hemoglobin A1c, glycated hemoglobin HOMA-IR, homeostasis model assessment of insulin resistance; BP, blood pressure. QUICKI, Quantitative Insulin sensitivity check index.
There were not significant differences in glycated hemoglobin, insulin, cortisol levels, HOMA-IR, and QUICKI between GDM patients and control women. However, pregnant women with GDM had higher ratio of triglycerides to high-density lipoprotein cholesterol (HDL) and lower HDL cholesterol than control women. Moreover, urea, aspartate aminotransferase, and fibrinogen levels were significantly higher in GDM patients than in control women. Besides, we also found significant differences in hemoglobin and leucocytes count in patients with GDM compared with normal pregnant women, although mean values were within normal limits (Table 1).
Sleep characteristicsNo significant differences in OSA symptoms between patients with GDM and control subjects were found (Table 2). There were no differences in self-reported sleep time on weekdays and reported naptime, but self-reported sleep time on weekends was slightly higher in patients with GDM than in the control group (Table 2). Minimum SaO2 was lower in the GDM group (90.8±5.3% vs. 92.3±3.1%, p=.027). Although mean and maximum obstructive apneas duration were longer in GDM patients, AHI and the remaining sleep parameters were similar among both groups (Table 3).
Sleep apnea related symptoms.
| Gestational diabetes mellitus (n=89) | Control pregnant women (n=88) | p value | |
|---|---|---|---|
| Snoring | 0.746 | ||
| Never (%) | 40.9 | 44.8 | |
| Sometimes (%) | 27.3 | 31.0 | |
| Usually (%) | 13.7 | 11.4 | |
| Always (%) | 18.2 | 12.6 | |
| Reported apnea | 0.327 | ||
| Never (%) | 87.1 | 94.2 | |
| Sometimes (%) | 10.6 | 5.8 | |
| Usually (%) | 1.2 | 0 | |
| Always (%) | 1.2 | 0 | |
| Frequent awakenings | 0.980 | ||
| Never (%) | 78.4 | 79.5 | |
| Sometimes (%) | 18.2 | 17 | |
| Usually (%) | 3.4 | 3.4 | |
| Always (%) | 0 | 0 | |
| Unrefreshing sleep | 0.696 | ||
| Never (%) | 43.7 | 36.5 | |
| Sometimes (%) | 33.3 | 32.9 | |
| Usually (%) | 17.2 | 21.2 | |
| Always (%) | 5.7 | 9.4 | |
| Morning headache | 0.846 | ||
| Never (%) | 85.2 | 82.8 | |
| Sometimes (%) | 12.5 | 12.6 | |
| Usually (%) | 2.2 | 3.4 | |
| Always (%) | 0 | 1.1 | |
| Nocturia | 0.328 | ||
| Never (%) | 13.8 | 11.4 | |
| Sometimes (%) | 14.9 | 14.8 | |
| Usually (%) | 25.2 | 38.7 | |
| Always (%) | 45.0 | 35.2 | |
| Sleepiness while driving | 0.185 | ||
| Never (%) | 96 | 91.1 | |
| Sometimes (%) | 4 | 8.9 | |
Sleep characteristics of the study subjects.
| Gestational diabetes mellitus (n=89) | Control pregnant women (n=88) | p value | |
|---|---|---|---|
| Epworth sleepiness scale | 6±3 | 7±3 | 0.614 |
| ASDA scale | 0.377 | ||
| No | 42.3 | 50.6 | |
| Mild | 49.3 | 35.8 | |
| Moderate | 7.0 | 11.1 | |
| Severe | 1.4 | 2.5 | |
| Reported sleep time/working days, h | 7.2±1.2 | 6.9±1.4 | 0.162 |
| Reported sleep time/weekends, h | 7.8±1.5 | 7.2±1.6 | 0.018 |
| Reported nap time/working days, mdaysmimin | 23.8±30.8 | 26.8±36.6 | 0.579 |
| Reported nap time/weekends, min | 38.3±38.4 | 31.4±40.6 | 0.253 |
| Total sleep time, min | 308±62 | 309±75 | 0.918 |
| Sleep efficiency, % | 72.3±14.1 | 70.3±15.8 | 0.388 |
| N1+N2 sleep time, % | 57.1±20.5 | 59.6±17.5 | 0.381 |
| N3 sleep time, % | 31.2±20.3 | 27.5±17.9 | 0.199 |
| REM sleep time, % | 11.7±5.7 | 13.0±5.4 | 0.133 |
| Apnea-hypopneas index, h−1 | 3.2±6.0 | 1.9±2.7 | 0.069 |
| Obstructive apneas duration, s | 6.4±15 | 2.7±6.2 | 0.034 |
| Maximum obstructive apneas length, s | 7.5±19 | 2.8±7.3 | 0.032 |
| Apnea-hypopneas duration, s | 20.9±14.9 | 20.8±14.4 | 0.981 |
| Maximum apnea-hypopneas length | 35.1±27.6 | 33.3±23.08 | 0.648 |
| Obstructive apneas index, h−1 | 0.5±2.3 | 0.07±0.18 | 0.112 |
| Central apneas index, h−1 | 0.16±0.49 | 0.13±0.29 | 0.708 |
| Mixed apneas index, h−1 | 0.09±0.3 | 0.04±0.2 | 0.241 |
| Arousal index, h−1 | 15.6±14.2 | 13.7±11.8 | 0.351 |
| Supine apnea-hypopneas index, h−1 | 2.7±4.7 | 2.5±4.0 | 0.875 |
| REM apnea-hypopneas index, h−1 | 7.3±15.5 | 4.1±7.1 | 0.080 |
| Mean SaO2, % | 96±2 | 96±1 | 0.813 |
| Minimum SaO2, % | 91±5 | 92±3 | 0.027 |
| T90, min | 0.48±2 | 0.66±2.9 | 0.640 |
| Desaturation index, h−1 | 1.0±3.0 | 0.7±1.5 | 0.287 |
Values represent mean±SD or percentage. Abbreviations: REM, rapid eye movement; SaO2, oxygen saturation; T90, total sleep time in minutes spent with SaO2<90%.
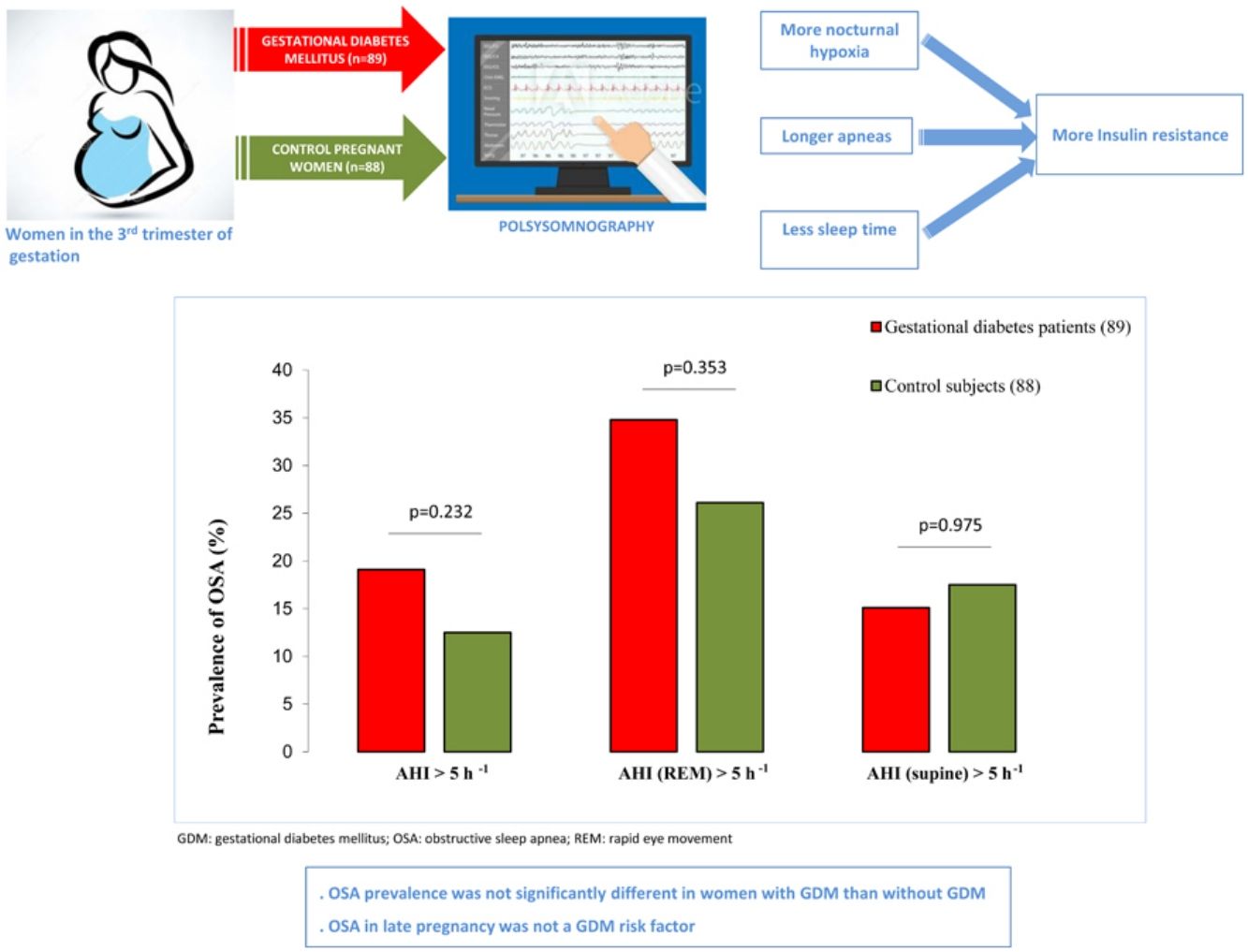
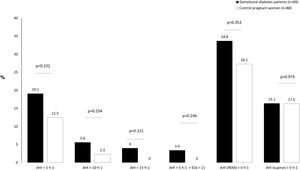
In the GDM group, 14.6% of women (n=13) had mild OSA, 3.4% (n=3) had moderate OSA, and 1.1% (n=1) had severe OSA. Among controls, 12.5% (n=11) had mild OSA. Fig. 2 shows the prevalence of an at least mild (AHI>5h−1), moderate (AHI>15h−1), and symptomatic (AHI>5h−1 and EES≥11) OSA in both groups. Besides, we illustrated the prevalence of REM and supine OSA. We did not find significant differences in percentages of associated OSA in any of the severity groups, neither in the specific supine OSA prevalence nor in the REM OSA prevalence among the study groups.
Association between OSA and GDMWe did not identify OSA as a GDM risk factor in the crude analysis. For several cutoffs, the AHI was not significantly associated with the presence of GDM (Fig. 2). An AHI>5h−1 had an odds ratio of 1.65 (95%CI: 0.73–3.77; p=.232) while an AHI in REM>5h−1 and an AHI in supine >5h−1 had crude odds ratios of 1.36 (95%CI: 0.71–2.58; p=.353) and 1.01 (95%CI: 0.43–2.39; p=.975), respectively. Additionally, the presence of an elevated AHI (>5h−1) and daytime hypersomnolence (ESS≥11) were not associated with GDM.
Relation between sleep characteristics and carbohydrate metabolismIn the overall study group, Glycated hemoglobin (hemoglobin A1c) was not related to sleep parameters. However, HOMA-IR, a surrogate marker of insulin resistance, was related to TST, REM sleep time, maximum duration of obstructive apneas, hypopneas index, AHI, maximum duration of apneas-hypopneas, T90, and DI (Table 4). Similarly, insulin sensitivity, which was assessed by QUICKI, was related with TST, arousal index, hypopnea index, AHI, maximum duration of apneas-hypopneas, and T90 (Table 4). All these variables, as well as age, gestational weight gain, pregestational body mass index, neck circumference, and waist–hip ratio, were included into a multiple regression model, which retained T90, TST, and maximum duration of respiratory events as independent factors related to HOMA-IR, while T90 was the only independent determinant of insulin sensitivity (Table 5).
Relationship between insulin resistance or sensitivity and sleep parameters in pregnant women, excluding patients with gestational diabetes mellitus with insulin treatment.
| Homa-IR | Quicki | |||||
|---|---|---|---|---|---|---|
| Correlation coefficient | p value | Correlation coefficient | p value | |||
| r | 95%CI | r | 95%CI | |||
| Total sleep time, min | −0.195 | −0.350 to −0.030 | 0.021 | 0.214 | 0.050 to 0.367 | 0.011 |
| REM sleep time, % | −0.167 | −0.324 to −0.001 | 0.049 | – | – | – |
| Arousal index, h−1 | – | – | – | −0.171 | −0.329 to −0.004 | 0.045 |
| Maximum obstructive apneas length, s | 0.219 | 0.052 to 0.374 | 0.011 | – | – | – |
| Hipopneas index, h−1 | 0.240 | 0.077 to 0.390 | 0.004 | −0.193 | −0.348 to −0.028 | 0.022 |
| Apnea–hypopneas index, h−1 | 0.241 | 0.078 to 0.391 | 0.004 | −0.194 | −0.349 to −0.029 | 0.022 |
| Maximum apnea–hypopneas length, s | 0.179 | 0.013 to 0.335 | 0.034 | −0.176 | −0.332 to −0.010 | 0.038 |
| T90, min | 0.358 | 0.204 to 0.495 | <0.001 | −0.220 | −0.372 to −0.056 | 0.009 |
| Desaturation index, h−1 | 0.210 | 0.046 to 0.363 | 0.013 | – | – | – |
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, qualitative insulin sensitivity check index; REM, rapid eye movement; SaO2, oxygen saturation; T90, total sleep time spent with SaO2<90%.
Independent predictors of insulin resistance and sensitivity in pregnant women.a
| Unstandardized regression coefficients | Standardized regression coefficients | p value | R2 | |||
|---|---|---|---|---|---|---|
| B | SE | 95%CI | B | |||
| HOMA-IR | ||||||
| T90, min | 0.189 | 0.044 | 0.102 to 0.277 | 0.453 | <0.001 | 0.139 |
| Total sleep time, min | −0.006 | 0.002 | −0.010 to −0.003 | −0.376 | 0.001 | 0.215 |
| Waist–hip ratio | −6.267 | 2.032 | −10.329 to −2.205 | −0.316 | 0.003 | 0.295 |
| Age, yr | −0.059 | 0.025 | −0.108 to −0.010 | −0.242 | 0.020 | 0.352 |
| Maximum apnea–hypopneas length, s | 0.008 | 0.004 | 0.000 to 0.015 | 0.216 | 0.038 | 0.396 |
| Constant | 11.974 | 2.386 | 7.204 to 16.743 | – | – | – |
| QUICKI | ||||||
| Pregestational BMI, kg/m2 | −0.002 | 0.001 | −0.003 to 0.000 | −0.257 | 0.027 | 0.097 |
| T90, min | −0.002 | 0.001 | −0.004 to 0.000 | −0.231 | 0.046 | 0.147 |
| Constant | 0.396 | 0.021 | 0.353 to 0.439 | – | – | – |
Abbreviations: HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, qualitative insulin sensitivity check index; T90, total sleep time spent with SaO2<90%; BMI, body mass index.
The main findings of the present study are: (1) OSA prevalence during the 3T was not significantly different in patients with GDM than in glucose-tolerant pregnant women. (2) We did not find significant associations between OSA and GDM. (3) HOMA-IR showed an inverse relationship with TST, and a direct correlation with maximum duration of apnea-hypopneas, while there was a direct relationship between T90 and HOMA-IR, and QUICKI.
Most previous studies that have evaluated the relationship between GDM and OSA were based on OSA symptoms,13 were retrospective,14,15 or used type 2–3 sleep devices.16 Moreover, there are some meta-analyses evaluating this association, but with conflicting findings.29–32 Taking into account all these studies, and its inconsistent conclusions, it is still unclear the existence of an association between OSA and GDM. The largest prospective study of OSA in pregnancy until now successfully performed home type 3 sleep tests to 2474 women at late second or early 3T. The prevalence of pre-pregnancy obesity was 23.3%, whereas OSA prevalence was 8.4% in mid-pregnancy. GDM was detected in 96 women, which was independently associated with OSA after adjustment for age, BMI, chronic hypertension, and weight gain (OR: 2.79, CI: 1.63–4.77).9 However, this study was designed and powered to determine if OSA was a risk factor for preeclampsia. Additionally, as a secondary objective, the authors aimed to examine the association with GDM. Furthermore, some women were classified as GDM using medical record abstraction when glucose tolerance test were not available. Nonetheless, the main limitation acknowledged by the authors was that OSA was diagnosed with a home sleep-recording device without EEG variables, that is not validated in pregnancy, which underestimate the burden and severity of the disease, and it does not allow to measure important variables like sleep architecture, arousals, or REM sleep apneic episodes.
To our knowledge, there are only four studies that have evaluated the potential association between GDM and OSA employing PSG recordings, and they deserve some comments. Louis et al.19 performed home PSG in obese pregnant women (76% Afro-American/Hispanic-American), and OSA prevalence was 15.4%. GDM was detected in 17 of them, but the prevalence of GDM was similar among OSA and non-OSA women. Similar results were found in a small case-control study that included 26 women with GDM. All participants underwent unattended home PSG during 3T. GDM women had significant higher ESS compared with control subjects, but neither AHI nor OSA prevalence were significantly different among study groups.18 On the contrary, Retrakul et al.20 found a higher prevalence of OSA in a group of 15 patients with GDM compared with 15 non-diabetic controls (73 vs 27%, p=.01). This study is also limited because of its small sample size and the inclusion of mostly non-Caucasian women with obesity. In keeping with these results, a recently small case-control study performed PSG in 46 women with GDM and in 46 controls, who were matched for age, gestational age, BMI, race, and parity.21 The frequency of OSA was found to be higher in GDM cases than in controls (22 vs. 9%). OSA was found to be significantly associated with the risk of GDM (OR, 1.81). Nevertheless, the main limitation of this study was that mean pre-pregnancy BMI was 30kg/m2, maybe because they over-included subjects with overweight/obesity. Furthermore, more than 75% of them were Afro-American/Hispanic-American. Obesity, and the high percentage of the previously mentioned ethnic groups in the study samples, which are known risk factors for both GDM and OSA, might have an impact on the main findings,1,33 which makes difficult any generalization of their results. As far as we know, our study has included the largest number of pregnant women studied by PSG in the evaluation of OSA as a risk factor for GDM to date. Overall, obesity rate was 10.2%, and, as expected, we found that women with GDM had a higher BMI than controls, which represents the usual clinical finding of patients with GDM. Despite this fact, we did not find that neither AHI nor prevalence of OSA were different among the study groups.
The relationships between GDM and OSA are complex, and any interactions between contributing factors are still unclear. Obesity is one of the main determinants to GDM risk. Besides, ethnicity and age could play major roles.1,22 In study of 105 pregnant women (75% African-American, BMI=33.4), a 27% of OSA frequency has been reported, moreover, with every BMI increase of 5kg/m2, subjects were nearly two times more likely to have OSA.34 Hence, larger studies with PSG are needed to better clarify the complex interrelationships between OSA and GDM, as well as the influence of important cofounding factors, such as obesity, ethnic background, and maternal age.
Specific hormonal and physiological changes during pregnancy could lead to OSA.7,8 The mechanisms responsible for the potential association with GDM have been poorly explained. Among others, it has been proposed that intermittent hypoxia, and arousals could lead to an increase in systemic inflammation, in oxidative stress, and in sympathetic activation, which could contribute to increase cortisol secretion, promoting IR, impairing glucose uptake, and increasing gluconeogenesis.7,10,11,16 However, most evidence of these multiple mechanistic pathways has yet to be demonstrated whether they are also involved during pregnancy.
We did not find significant associations between arousal index and IR. Moreover, we did not find differences in AHI, but patients with GDM had longer mean and maximum obstructive apneas length, in addition to lower nocturnal minimum SaO2. Besides, HOMA-IR was related to maximum duration of respiratory events, and both HOMA-IR and QUICKI were directly proportional to CT90 independently of obesity. Longer sleep event duration has been previously associated with lower nocturnal SaO2 and longer CT90 in OSA.35 Similarly, other groups showed that nocturnal hypoxia markers correlated positively with fasting glucose levels,20,36 IR, and beta cell dysfunction.37 Based on these findings, nocturnal hypoxia could be a potential intermediate mechanism and a better factor in the complex relationship between glucose metabolism and OSA during pregnancy.38 However, a limited number of studies with very small samples, and some confounding variables have examined whether nocturnal hypoxia increases the risk of IR. The percentage of women with obesity was high in most studies, and it could also be possible that the increased risk may be related to obesity or other comorbidities and not to OSA per se.
Sleep time decreases during gestation, particularly by the 3T. Additionally, multifactorial aspects such as psychosocial, ethnic, physical, and biological factors predispose to worsen the sleep quality.17,39 There is some evidence that shorter sleep time is associated with higher glucose levels and GDM.40 We also found that objectively shorter TST was independently associated with higher HOMA-IR. Consequently, an alternative hypothesis is that sleep duration, as well as sleep quality not related to OSA, increases sympathetic activity41 and results in hypothalamic-pituitary-adrenal axis dysregulation,42 which could promote IR. Nonetheless, TST was based in only one night evaluation, and further studies are needed to explore this hypothesis.
Strengths of our study include a multicenter approach, a prospective enrolment, sufficient sample size, and the use of the same protocol in both cases and controls with an objective measure of sleep parameters with PSG performed at hospital. Additionally, laboratory researchers and study personnel were blinded to maternal status. Yet, a number of potential limitations deserve comment. First, the global refusal rate to participate was high (29%), which was though expectedly, as previous studies showed a similar and even much higher percentage of refusal rate (26–88%) both in GDM patients,18,19 and in other groups of pregnant women.7,30 The Institutional Ethic Committee did not allow the collection of information from those women who had declined participation, however we believe that the consecutive recruitment of patients, as well as the general and anthropometric characteristics of the selected women, similar to series of both GDM and healthy pregnant women from the same geographical area,43–45 suggest that the study sample could be considered as representative and makes it difficult to consider a selection bias. Second, as it is already known, we found patients with GDM had higher pregestational BMI. However, it is unlikely that this would modify our results to any important extent since obesity prevalence was relatively low (10%), and despite this, we did not find differences neither in AHI nor in OSA prevalence between both study groups. Third, since episodes of flow limitation <30% associated with arousal were not scored, we cannot ascertain whether our results would still be similar, though, it is unlikely to affect our conclusions as previous studies evaluating OSA in GDM and integrating flow limitation scoring, showed no remarkable influence, since the sleep time spent with flow limitation was similar in both groups in one of the studies,18 while the other study found that the flow limitation index was not associated with increased risk of GDM, spite of the fact the authors showed that OSA prevalence (based only in AHI) was significantly higher in GDM than in control pregnant women.21 Fourth, because we included mainly Caucasian women, our results may not be directly applicable to other ethnic groups. Fifth, further studies are required to better determine the influence of OSA, since the women with OSA of the present study mainly had very mild forms, which could limit further analysis to study the association between DGM and moderate-severe OSA. Finally, the design of the present study does not allow to determine whether OSA started previously or during early or late pregnancy.
ConclusionIn summary, our results did not demonstrate significant differences in OSA prevalence among patients with GDM and control pregnant women, and we did not find significant and independent associations between OSA and GDM. Nevertheless, we found that IR was negatively related with TST and positively related to respiratory apnea-hypopneas length, as well as nocturnal hypoxia, representing that OSA influence on IR during pregnancy could be more complex than its effect on AHI would implicate. The number of studies that have evaluated the interactions of OSA with GDM is still scarce, with major heterogeneity in research design, and populations included. Further, larger, multicenter, and more rigorous investigations employing PSG studies in all subjects, with attention to important confounding factors such as obesity, age and ethnic background, are clearly needed to better clarify and characterize the potential complex interrelationships between OSA and GDM,46 in the meantime, practitioners should screen and treat OSA in suspected pregnant women.
FundingThis research was partially supported by grants from SEPAR-2010-820, Ministerio de Economía y Competitividad (PI10/00495), Ministerio de Ciencia, Innovación y Universidades (PI19/00875), and Programa “Ramon Llull”, contractes per a la Intensificació de l’Activitat Investigadora a l’IdISBa 2020.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank all women for their willingness to participate. The authors thank Meritxell-Arqué, for her assistance in the study fieldwork, and J. Rebolo-Roca for his language assistance.