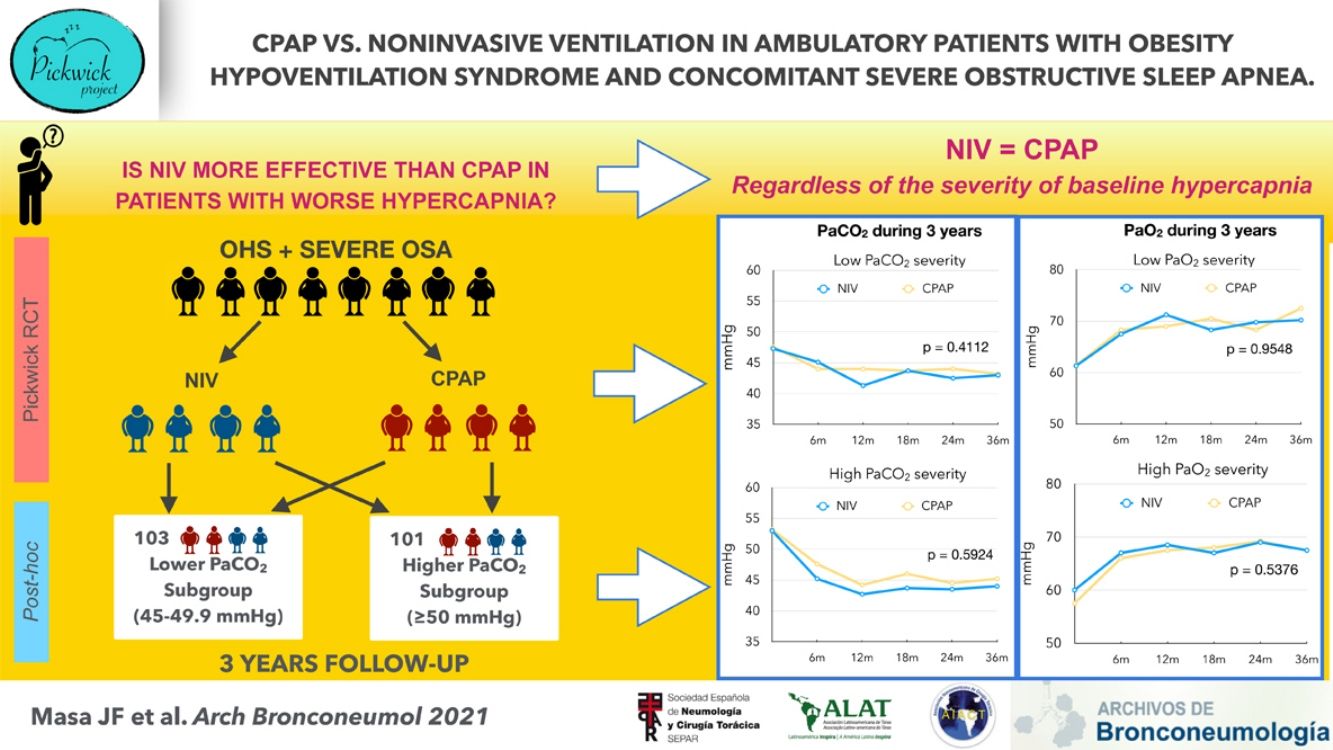
Obesity hypoventilation syndrome (OHS) with concomitant severe obstructive sleep apnea (OSA) is treated with CPAP or noninvasive ventilation (NIV) during sleep. NIV is costlier, but may be advantageous because it provides ventilatory support. However, there are no long-term trials comparing these treatment modalities based on OHS severity.
ObjectiveTo determine if CPAP have similar effectiveness when compared to NIV according to OHS severity subgroups.
MethodsPost hoc analysis of the Pickwick randomized clinical trial in which 215 ambulatory patients with untreated OHS and concomitant severe OSA, defined as apnoea-hypopnea index (AHI)≥30events/h, were allocated to NIV or CPAP. In the present analysis, the Pickwick cohort was divided in severity subgroups based on the degree of baseline daytime hypercapnia (PaCO2 of 45–49.9 or ≥50mmHg). Repeated measures of PaCO2 and PaO2 during the subsequent 3 years were compared between CPAP and NIV in the two severity subgroups. Statistical analysis was performed using linear mixed-effects model.
Results204 patients, 97 in the NIV group and 107 in the CPAP group were analyzed. The longitudinal improvements of PaCO2 and PaO2 were similar between CPAP and NIV based on the PaCO2 severity subgroups.
ConclusionIn ambulatory patients with OHS and concomitant severe OSA who were treated with NIV or CPAP, long-term NIV therapy was similar to CPAP in improving awake hypercapnia, regardless of the severity of baseline hypercapnia. Therefore, in this patient population, the decision to prescribe CPAP or NIV cannot be solely based on the presenting level of PaCO2.
El síndrome de hipoventilación-obesidad (SHO) con apnea obstructiva del sueño (AOS) grave concomitante se trata con CPAPo ventilación no invasiva (VNI) durante el sueño. La VNI es más costosa, pero puede ser beneficiosa porque proporciona soporte ventilatorio; sin embargo, no existen estudios a largo plazo que comparen estas modalidades de tratamiento basándose en la gravedad del SHO.
ObjetivoDeterminar si la CPAP tiene una eficacia similar a la VNI según los subgrupos de gravedad del SHO.
MétodosAnálisis a posteriori del ensayo clínico aleatorizado Pickwick en el que 215 pacientes ambulatorios con SHO sin tratar y con AOS grave concomitante (definida como un índice de apnea-hipopnea [IAH] ≥ 30 episodios/hora) recibieron tratamiento con VNI o CPAP. En el presente análisis, la cohorte Pickwick se dividió en subgrupos según la gravedad basándose en el grado de hipercapnia diurna al inicio del estudio (PaCO2 de 45-49.9mm Hg o ≥ 50mm Hg). Se compararon las mediciones periódicas de PaCO2 y PaO2 durante los 3 años siguientes entre la CPAP y la VNI entre los dos subgrupos de gravedad. Se realizó un análisis estadístico utilizando un modelo lineal mixto.
ResultadosSe analizaron 204 pacientes, 97 en el grupo de VNI y 107 en el grupo de CPAP. Las mejoras lineales de PaCO2 y PaO2 fueron similares entre la CPAP y la NIV según los subgrupos de gravedad en función de la PaCO2.
ConclusiónEn los pacientes ambulatorios con SHO y AOS grave concomitante a los que se trató con VNI o CPAP, el tratamiento a largo plazo con VNI resultó similar a la CPAP, en cuanto a la mejora de la hipercapnia en vigilia, independientemente de la gravedad de la hipercapnia de inicio. Por lo tanto, en esta población de pacientes la decisión de prescribir CPAP o VNI no puede basarse exclusivamente en el nivel de partida de PaCO2.
Obesity hypoventilation syndrome (OHS) is defined by obesity, hypercapnia during wakefulness, and sleep-disordered breathing, without other causes of hypoventilation.1 Roughly 90% of patients with OHS have concomitant obstructive sleep apnea (OSA),2 with 73% having severe OSA.3 Compared with eucapnic OSA4,5 and eucapnic obese,6,7 patients with OHS have a higher prevalence of cardiovascular and respiratory morbidity resulting in increased hospitalization risk, greater healthcare resource utilization, and augmented mortality.7–16
Ambulatory patients with OHS are typically treated with nocturnal positive airway pressure (PAP) therapy during sleep such as noninvasive ventilation (NIV), usually with bilevel pressure settings, or continuous positive airway pressure (CPAP). The effectiveness of NIV has been evaluated in several long-term, observational studies;4,6,7,16–23 and medium-term randomized trials.3,24,25 CPAP prevents upper airway obstructive events, but it is not the treatment of choice for non-obstructive sleep hypoventilation.21 Several medium-term clinical trials3,26,27 and one long-term28 randomized clinical trial have reported similar outcome between NIV and CPAP. CPAP is simpler to implement and is less costly than NIV,28 therefore, CPAP has been proposed as first-line treatment in OHS and concomitant severe OSA.28,29 In the largest randomized clinical trial to date (Pickwick study), the longitudinal improvement in arterial blood gases (ABG) were similar between NIV and CPAP.28
There is no consensus on how to define OHS severity. Classification of OHS severity based on PaCO2, OSA severity, or associated comorbidities have been proposed.30–32 However, there are no randomized clinical trials or prospective longitudinal studies assessing the effectiveness of PAP treatment (NIV and CPAP) based on any of the previously proposed OHS severity classifications. Higher PaCO2 has been related to mortality17,33,34 and reflects the consequences of potential mechanisms underlying chronic hypercapnic respiratory failure in OHS.35–37 We hypothesized that in ambulatory patients with OHS and concomitant severe OSA, long-term NIV therapy will lead to a greater improvement in awake PaCO2 and PaO2 than CPAP in the subgroup with more severe daytime hypercapnia at baseline. To test this hypothesis, we performed a post hoc analysis of the Pickwick study.3,25,28,38–44 We examined the longitudinal changes in arterial blood gases (PaCO2 and PaO2) in patients with OHS and concomitant severe OSA undergoing long-term therapy with NIV or CPAP. We classified patients into two OHS severity subgroups using baseline daytime PaCO2: PaCO2 of 45–49.9 or ≥50mmHg.
MethodsTrial designWe carried out a multicenter, open-label, randomized clinical trial with two parallel-groups conducted at 16 clinical sites in Spain.
ParticipantsFrom May 2009 to March 2013, we successively screened ambulatory patients between 15 and 80 years of age who were referred for pulmonary consultation due to suspected OHS or OSA at 16 tertiary hospitals in Spain (see online supplement). OHS was defined as obesity, with a body mass index (BMI)≥30kg/m2, stable hypercapnic respiratory failure (PaCO2≥45mmHg, pH≥7.35, and no clinical exacerbation during the previous two months), no noteworthy spirometric evidence of chronic obstructive pulmonary disease [forced expiratory volume in the first second (FEV1) had to be above 70% of predicted in cases where FEV1/forced vital capacity (FVC) was below 70], and no evidence of neuromuscular, chest wall, or metabolic disease to explain hypercapnia. Other inclusion criteria were as follows: (1) severe OSA [apnea-hypopnea index (AHI)≥30events/h], (2) an absence of narcolepsy or restless legs syndrome, and (3) a correctly executed 30-min CPAP/NIV treatment test (see online data supplement). The exclusion criteria were as follows: (1) a psycho-physical inability to complete questionnaires, (2) severe chronic debilitating illness, (3) severe chronic nasal obstruction, and (4) a lack of informed consent.
The Pickwick project comprised two parallel randomized clinical trials conducted in two phases39 (see online supplement). The study was approved by the 16 centers ethics committees, and written informed consent was obtained from all patients.
InterventionsAmbulatory OHS patients with concomitant severe OSA (AHI≥30) were randomized by an electronic database without pre-set allocation rate (simple randomization) into NIV, CPAP, or control group for two months (first phase). After this period, patients in the control group (i.e. lifestyle changes) were re-randomized to NIV or CPAP by a simple randomization due to pre-specified ethical concerns of not treating patients with OHS and severe OSA beyond two months. All patients randomized to NIV or CPAP were followed for 3 years (second phase). Both arms (CPAP and NIV) were additionally treated with lifestyle recommendations (see online supplement). Oxygen therapy was added if baseline daytime (PaO2 was <55mmHg) or nocturnal hypoxemia (mean SpO2 from the pulse-oximetry signal during the titration PSG was ≤88%) was detected during PAP titration.41
CPAP titrationPatients were instructed to use at-home fixed CPAP during the entire sleep period based on a conventional polysomnographic CPAP titration (see online supplement).
NIVPatients were instructed to use NIV treatment during the entire sleep period. The ventilator mode was set at a bi-level PAP with assured volume (see online data supplement).
Masking strategyThe trial was open-label for both the investigators and the patients. However, the treating clinicians (routine care team) were uninformed of the research study (see online data supplement).
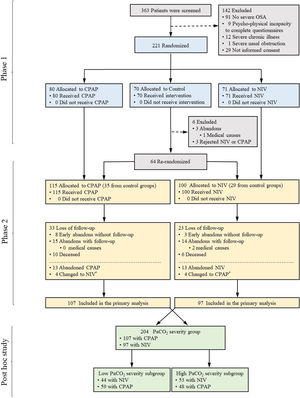
OutcomesPatients were evaluated on 12 occasions during 3 years: at baseline, at 2 months, at six months and every three months until completing 3 years of follow-up. Evaluations at the second month were performed before re-randomization of the control group to CPAP or NIV and therefore, it was not included in the current analysis3 (Fig. 1).
Flow chart of the study protocol. Of 363 selected patients, 142 were excluded and 221 were randomized. After the first two months of treatment (first phase), patients included in the control group (i.e. lifestyle changes) were re-randomized to CPAP or NIV in order to be followed for three years (second phase). 215 patients were randomized to either CPAP (n=115) or NIV (n=100). From the 115 patients included in the CPAP arm, 8 abandoned the study early without follow-up and the rest (n=107) were available for the primary analysis. From the 100 patients included in the NIV arm, 3 abandoned the study early without follow-up and the rest (n=97) were available for the primary analysis. The present post hoc study included these 204 available patients to be distributed in four severity subgroups (low and high PaCO2 and low and high AHI severity subgroups). *=Patients who changed treatment after randomization (i.e. from CPAP to NIV or vice versa) were analyzed in the original arm according to the intention-to-treat principle.
At baseline visit we assessed anthropometric data, smoking habits, alcohol consumption, arterial blood gases (ABG) on room air to assess PaCO2, PaO2 (see online data supplement), spirometry45 six-minute walk distance (6-MWD) test,46 dyspnea by Medical Research Council (MRC) scale, sleepiness on the Epworth Sleepiness Scale (ESS), comorbidities (hypertension, diabetes, dyslipidemia, ischemic cardiomyopathy, chronic heart failure, stroke, pulmonary hypertension, cardiac arrhythmia, and leg arteriopathy) obtained from the electronic medical records and during face-to-face interview with patients, health related quality of life tests using the Functional Outcomes of Sleep Questionnaire (FOSQ) and the Medical Outcome Survey Short Form 36 (SF 36), oxygen therapy and conventional polysomnography (see online supplement). In the subsequent visits, we performed ABG on room air and assessment of adherence to CPAP/NIV using internal device hourly counters. During all visits, we encouraged treatment adherence and made adjustments to supplemental oxygen therapy or PAP settings and masks if the patients required them. The trial was stopped when the last enrolled patient completed 3 years of follow-up.
Classification of OHS severityGiven the lack of consensus on how to define OHS severity, in this post hoc analysis we classified patients enrolled in the Pickwick trial into two clinical severity subgroups using a PaCO2 cut-off point of 50mmHg.31 Consequently, we constructed two subgroups: (1) high PaCO2 (≥50mmHg); and (2) low PaCO2 (<50mmHg).
Statistical analysisBaseline bivariate analysis was carried out by t-test (or equivalent non-parametric) or χ2 test depending on quantitative or categorical variables, respectively between CPAP and NIV in two severity subgroups. Other bivariate analysis was also performed between high vs. low PaCO2 severity subgroups, regardless of CPAP or NIV treatment. The normality of the distribution was analyzed using the Shapiro–Wilk test. Repeated measures of ABG parameters (PaCO2 and PaO2) during 3 years of follow up were compared between CPAP and NIV in each severity subgroup. Linear mixed-effects models were used to analyze longitudinal ABG outcomes. The models included treatment (CPAP and NIV), visit and treatment/visit interaction as fixed effects and the subjects as random effects. The visit was considered as a categorical factor.47 Furthermore, time until the visit as a continuous variable was used for linear evolution test. Because we analyzed subgroup of patients from the original randomization with more probability of arm imbalances, we planned to adjust all models for potential confounding factors such as age, sex, smoking habits, body mass index (BMI), adherence and outcomes from bivariate analysis with p<0.05, if these existed. PAP usage of more than 4h per day was considered adequate adherence to PAP therapy.28 Additional longitudinal assessment of body weight in the two severity subgroups between CPAP and NIV was analyzed similarly as described for ABG outcomes. Other longitudinal models added baseline values of weight, PaCO2 and PaO2 as adjusted variables to analyze the evolution of these parameters after baseline evaluation (from 6 months to 3 years).
Data management and statistical analyses were performed using R software (R Core Team 2017, Version 3.4.2, Vienna, Austria) and SPSS software (IBM-SPSS Statistics, Version 22.0. Armonk, NY: IBM Corp.).
ResultsStudy participantsThe Pickwick trial was performed in two phases that included 221 patients (Fig. 1, Phase 1). For the long-term study (Fig. 1, Phase 2), 115 patients were allocated to CPAP and 100 to NIV. Of these 215 patients, 204 were available for longitudinal analysis, 97 patients for the NIV group and 107 for the CPAP group. Of these 204 patients (97 with NIV and 107 with CPAP), 103 (44 with NIV and 59 with CPAP) were included in the low PaCO2 severity subgroup and 101 (53 with NIV and 48 with CPAP) were included in the high PaCO2 severity subgroup (Fig. 1, Post hoc study). For the entire cohort, the median [IQR] follow-up was 3.00 [2.92;3.17] years for the CPAP group and 3.00 [2.92;3.08] years for NIV group. Table 1 describes the characteristics at baseline and PAP adherence between high and low PaCO2 severity subgroups. The high PaCO2 severity subgroup had worse quality of life, ABG, spirometry, mean sleep time oxygen saturation and sleep time with SpO2<90%, and had a higher prevalence of hypertension than the low PaCO2 severity subgroup. Table E1 describes the global characteristics of the population. Table E2 compares baseline characteristics between treatment groups (NIV or CPAP) in each severity subgroups (low vs. high PaCO2). No significant differences were found between CPAP and NIV in any of the subgroups.
Characteristics of patients at baseline according PaCO2 and AHI severity groups.a
| PaCO2 severity | |||
|---|---|---|---|
| Low-severity | High-severity | p value | |
| N=103 | N=101 | ||
| Age, years | 61.0 [51.0;69.0] | 65.0 [56.0;72.0] | 0.077 |
| Sex, male | 51 (49.5%) | 38 (37.6%) | 0.116 |
| Smokers | 30 (29.1%) | 21 (20.8%) | 0.388 |
| Smoking, pack/yearb | 25.0 [15.0;40.0] | 36.0 [15.0;49.5] | 0.182 |
| Drinkersc | 24 (23.3%) | 15 (14.9%) | 0.175 |
| Alcohol, grb | 30.0 [20.0;57.0] | 30.0 [13.0;40.0] | 0.265 |
| BMI, kg/m2 | 41.6 [38.0;47.1] | 44.3 [38.4;49.6] | 0.094 |
| Neck circumference, cm | 44.4 (4.48) | 44.7 (4.79) | 0.643 |
| ESS | 10.9 (4.94) | 11.0 (5.24) | 0.921 |
| FOSQ | 78.5 [62.0;92.5] | 68.0 [53.0;84.8] | 0.003 |
| SF 36-Physical | 38.9 [29.9;46.0] | 32.9 [27.7;41.8] | 0.046 |
| SF 36-Mental | 45.3 [30.4;53.1] | 45.6 [33.5;53.0] | 0.354 |
| Dyspnea MRC scale≥2 | 55 (53.4%) | 64 (63.4%) | 0.193 |
| Hypertension | 63 (61.2%) | 77 (76.2%) | 0.030 |
| Diabetes | 39 (37.9%) | 37 (36.6%) | 0.971 |
| Dyslipidemia | 40 (38.8%) | 50 (49.5%) | 0.163 |
| Heart failure | 9 (8.82%) | 9 (8.91%) | 1.000 |
| Stroke | 16 (15.5%) | 14 (13.9%) | 0.889 |
| Arrhythmia | 9 (8.74%) | 7 (6.93%) | 0.826 |
| Ischemic heart disease | 10 (9.71%) | 7 (6.93%) | 0.642 |
| Leg arteriopathy | 8 (7.77%) | 2 (1.98%) | 0.101 |
| Pulmonary hypertension | 9 (8.82%) | 8 (7.92%) | 1.000 |
| pH | 7.41 [7.38;7.43] | 7.39 [7.37;7.41] | 0.010 |
| PaO2, mmHg | 63.5 [57.4;70.0] | 58.0 [54.0;64.0] | <0.001 |
| PaCO2, mmHg | 47.2 [46.3;48.4] | 53.0 [51.0;56.0] | <0.001 |
| Bicarbonate, mmol/l | 28.1 [27.0;30.2] | 30.6 [28.6;33.0] | <0.001 |
| FEV1in % of predicted | 81.0 [71.2;92.0] | 73.0 [61.0;84.0] | <0.001 |
| FVC, in % of predicted | 83.5 [72.2;96.0] | 76.0 [64.0;85.0] | 0.001 |
| 6-MWD in meters | 386 [304;480] | 360 [243;430] | 0.066 |
| Polysomnographic parameters | |||
| TST, h | 5.29 (1.31) | 5.30 (1.30) | 0.942 |
| non-REM stage 1 and 2, % | 85.4 [74.8;91.4] | 84.7 [73.0;92.1] | 0.753 |
| non-REM stage 3, % | 5.00 [0.00;11.6] | 4.19 [0.00;16.1] | 0.964 |
| REM sleep % | 7.00 [2.50;14.2] | 8.20 [4.50;13.8] | 0.656 |
| Arousal index | 53.0 [31.2;73.6] | 59.1 [32.5;85.3] | 0.243 |
| AHI | 68.3 [45.0;92.8] | 68.7 [43.2;96.4] | 0.892 |
| ODI | 69.9 [40.9;93.0] | 73.2 [44.5;95.0] | 0.721 |
| Mean SpO2 | 86.7 [83.0;90.0] | 84.0 [78.8;87.1] | 0.002 |
| TST with SpO2<90%, % | 70.0 [45.1;92.4] | 82.5 [56.2;96.7] | 0.010 |
| PAP adherence (>4h/day) | 65 (63.1%) | 68 (67.3%) | 0.627 |
| Oxygen therapy | 20 (19.4%) | 30 (29.7%) | 0.122 |
| Oxygen therapy flow, L/minb | 1.50 [1.50;2.00] | 1.75 [1.12;2.00] | 0.806 |
People who drink more than 30g of alcohol/day in men and 20g in women.
Abbreviations: SD=standard deviation; IQR=interquartile range; BMI=body mass index; EES=Epworth sleepiness scale; MRC=Medical Research Council; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; 6-MWD=six-minute walk distance; TST=total sleep time; AHI=apnea-hypopnea index; ODI=3% oxygen desaturation index; and SpO2=oxygen saturation by pulse oximetry.
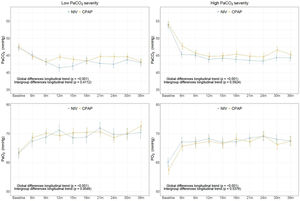
In the high PaCO2 severity subgroup, PaCO2 and PaO2 improved significantly with PAP, but without any statistically significant differences between NIV and CPAP (Fig. 2 and Table E3). Similarly, in the low PaCO2 severity subgroup, ABG parameters improved significantly with both treatments without groups differences, except in the individual comparison at 12 months (Table E4). In the model with adjustment for baseline values of ABG parameters, there was no significant difference in PaCO2 and PaO2 between CPAP and NIV. In both treatment groups, PaO2 improved from 6 months to 36 months in the low PaCO2 severity subgroup (Fig. E2).
Adjusted longitudinal changes of ABG parameters (mean and SE) from linear mixed-effects models during the follow-up related to intervention treatment groups in low and high PaCO2 severity subgroups. p values correspond to adjusted (adjusted by age, sex smoking status, body mass index (BMI) and adherence) longitudinal changes for ABG and for the inter-group CPAP and NIV comparison. Abbreviations: ABG=arterial blood gases; CPAP=continuous positive airway pressure; NIV=noninvasive ventilation; and SE=standard error.
In both groups of severity, weight was significantly reduced overtime with either CPAP or NIV treatment. However, longitudinal weight change was similar between CPAP and NIV (Fig. E3 and Table E5). In the model with adjustment for baseline weight, there was no significant difference between CPAP and NIV in the two severity subgroups (Fig. E4).
DiscussionThis post hoc analysis of the largest multi-center clinical trial with the longest period of follow-up in adult patients with OHS is the first to examine long-term outcomes based on the severity of OHS. The study included ambulatory patients with OHS and concomitant severe OSA who were in stable chronic hypercapnic respiratory failure. Our analysis shows that there were no significant long-term differences in the improvement of daytime PaCO2 and PaO2 between NIV and CPAP based on whether the baseline PaCO2 was between 45–49.9 or ≥50mmHg.
Conceptually, CPAP is not a treatment for nocturnal hypoventilation that is not a result of upper airway obstructive events.21,24,48 Consequently, the Pickwick study was designed to compare CPAP with NIV in OHS patients with severe OSA (AHI≥30) because CPAP can be less effective than NIV in patients with an AHI<30. This approach has been endorsed by the American Thoracic Society clinical practice guidelines.29
In addition to the severity of OSA, several other factors may influence clinicians to select NIV over CPAP in stable ambulatory patients with OHS and severe OSA. Some factors that may favor the prescription of NIV include higher baseline PaCO2 and persistent hypoxemia or hypercapnia during CPAP titration (i.e. CPAP titration failure). In this context, NIV would seem more appropriate for patients with more severe hypercapnia. Although several clinical trials have shown no significant difference in outcomes between CPAP and NIV,28,49 it remains unclear whether there is a subset of patients who would benefit more from NIV than CPAP. This is clinically relevant because an in-laboratory CPAP titration failure does not necessarily mean long-term CPAP treatment failure, suggesting that even an in-laboratory PAP titration study may not ultimately be able to distinguish patients who will finally fail long-term CPAP therapy.50 Higher PaCO2 has been associated with higher mortality.17,33–37 In OHS patients with severe OSA, awake baseline PaCO2 seems to be a reasonable marker of OHS severity (Table 1). We chose longitudinal ABG parameters as the outcome because in clinical practice, ABG is the most frequent and available objective test for medium and long-term assessment.
An observational retrospective study compared the effectiveness of PAP according to severity groups using baseline daytime PaCO2 in OHS.31 CPAP responders (who achieved oxygen saturation during sleep ≥90%, together with resolution of obstructive events) during polysomnographic CPAP titration were treated with CPAP and the remaining with NIV. CPAP responders were more frequently in the lowest severity group of baseline PaCO2 compared with the highest severity group, suggesting that clinicians may use baseline PaCO2 or the degree of persistent hypoxemia during CPAP titration as reasons to prescribe NIV. However, CPAP titration failure does not necessarily mean long-term CPAP treatment failure50 and results from several randomized clinical trials of unselected patients do not support this approach.3,27,28 Although some studies have observed a medium-term delay in CPAP effectiveness in comparison with NIV,3,27,28,38,43 long-term outcomes do not differ between CPAP and NIV in the subset of patients with OHS who also have co-existent severe OSA. The present study provides evidence that in patients with OHS and concomitant severe OSA (nearly 70% of all patients with OHS), a higher level of baseline hypercapnia is not a justification to recommend NIV over CPAP.
Short-term studies have demonstrated a significant association between CPAP or NIV adherence and improvement in daytime hypercapnia.3,51 Higher level of adherence to either NIV or CPAP (>4h per day) has been associated with long-term outcomes as lower hospital resource utilization and mortality in the Pickwick cohort.28 For this reason we included adherence in our longitudinal model despite the fact that adherence was similar between CPAP and NIV in the two severity subgroups (Table E2).
In the long-term Pickwick trial,28 body weight was reduced significantly during follow-up without differences between CPAP and NIV. We have included in the present analysis the longitudinal change in body weight in the two severity subgroups given that weight loss (or weight gain) may impact our outcome of PaCO2 and PaO2. Since there was no significant difference in the degree of weight change between CPAP and NIV, the potential influence of weight reduction on ABG parameters should be similar for CPAP and NIV.
Our study has several limitations. First and foremost, our results represent a post hoc analysis of the original randomized controlled trial and as such, the current analysis was a non-specified subgroup analysis. Importantly, by creating 2 subgroups, the statistical power is reduced. We chose a cut-off point of 50mmHg for the baseline PaCO2 to create two subgroups of OHS severity because it is a value frequently used in clinical practice and because in another study, this cut-off point discriminated between groups with higher and lower oxygenation level during sleep.31 However, it is possible that other cutoff points may lead to different conclusions. As such, our results should be interpreted with caution. Our cohort was limited to ambulatory OHS patients who also have concomitant severe OSA. Since all patients in this study had severe OSA, defined as an AHI≥30, we could not reliably classify patients into subgroups of OSA severity because of an AHI floor effect. As such, it is important to note that our findings do not apply to the minority of patients with OHS who do not have concomitant severe OSA (∼30% of patients with OHS). Our external validity may be limited because the study was performed exclusively in Spain and patients from other ethnic/racial backgrounds may have different characteristics, which may impact the results. Finally, we did not use transcutaneous CO2 as a direct measure of hypoventilation during polysomnographic PAP titration, although we increased the inspiratory positive airway pressure (IPAP) during NIV titration to achieve the best oxygenation level as a surrogate marker of hypoventilation.3
In conclusion, in patients with stable OHS and concomitant severe OSA, NIV and CPAP have similar long-term effectiveness in improving PaCO2 and PaO2 irrespective of OHS severity based on baseline hypercapnia. Therefore, clinicians can initiate CPAP treatment regardless of PaCO2 severity in this patient population. A randomized clinical trial focused on using various definitions of OHS severity is needed to determine in which patients NIV would lead to better long-term outcomes.
Role of the sponsorsThe sponsors and funders of the study had no involvement or any influence in study design, in the collection, analysis, and interpretation of data, in writing of the manuscript, and in the decision to submit the manuscript for publication. The corresponding author (JFM) confirms that he had full access to all the data and he had the final responsibility for the decision to submit for publication.
Data sharing statementAdditional related documents such as study protocol, statistical analysis plan, and informed consent form will be available upon request from the Pickwick Project principal investigator (Dr. Juan Fernando Masa). Deidentified patients’ data can be requested by researchers for use in independent scientific research and will be provided following review and approval of the research proposal (including statistical analysis plan) and completion of a data sharing agreement with the Pickwick Project Publications Committee. Investigator Data requests can be made anytime from 1 to 2 years after the publication of this trial. Requests should be sent to the corresponding author (Dr. Juan Fernando Masa – fmasa@separ.es).
Authors’ contribution1. Substantial contributions to study conception and design, acquisition of data, or analysis and interpretation of data: Juan F. Masa, MD, PhD, M-Ángeles Sánchez-Quiroga, MD, Francisco Javier Gomez de Terreros, MD, PhD, Iván Benítez, BSc(Stat), Francisco-José Vázquez-Polo PhD; Miguel A. Negrín PhD; María Martel-Escobar PhD; Ferran Barbe, MD, PhD, Auxiliadora Romero, MD, Candela Caballero-Eraso MD, PhD, Teresa Gomez-Garcia, MD, Mónica González, MD, PhD, Soledad López-Martín, MD; José M Marin, MD, PhD; Odile Romero, MD; Trinidad Díaz-Cambriles, MD, Eusebi Chiner, MD, PhD, Carlos Egea, MD, PhD, Babak Mokhlesi, MD, MSc, Jaime Corral, MD, Galo Fernandez, MD, Javier Barca, MD, Estrella Ordax, MD, Nicolás González-Mangado, MD, PhD, Maria F Troncoso, MD, Maria-Ángeles Martinez-Martinez, MD, Elena Ojeda-Castillejo, MD; Daniel López Padilla MD, Santiago J. Carrizo, MD, PhD, Begoña Gallego, MD, PhD, Mercedes Pallero, MD, Odile Romero MD,Sergi Martí, MD, PhD, Maria Antonia Ramón, PT, MSc, Eva Arias, MD, Jesús Muñoz-Méndez, MD, PhD, Cristina Senent, MD; Jose N. Sancho-Chust, MD, Nieves Belén Navarro Soriano, MD, Emilia Barrot MD, PhD, José M. Benítez, MD, Jesús Sanchez-Gómez, MD, Rafael Golpe, MD, PhD, Rocio Gallego, MD, Juan A Riesco, MD, Ana Santiago-Recuerda, MD, PhD; Silvia Gomez, MD, and Mónica Bengoa, MD.
2. Drafting the article or revising the article critically for important intellectual content: Juan F. Masa, MD, PhD, Babak Mokhlesi, MD, MSc, Iván Benítez, BSc(Stat), Francisco Javier Gomez de Terreros, MD, PhD, Maria Ángeles Sánchez-Quiroga, MD, Auxiliadora Romero, MD, Candela Caballero-Eraso MD, PhD, Maria F. Troncoso, MD, Mónica González, MD, PhD, Soledad López-Martín, MD, José M. Marin, MD, PhD, Sergi Martí, MD, PhD, Trinidad Díaz-Cambriles, MD, Eusebi Chiner, MD, PhD, Carlos Egea, MD, PhD, Francisco-José Vázquez-Polo PhD; Miguel A. Negrín PhD; María Martel-Escobar PhD; Ferran Barbe, MD, PhD and Jaime Corral, MD.
3. Performing of the version to be published: Juan F. Masa, MD, PhD, Babak Mokhlesi, MD, MSc, Ivan Benitez, BSc(Stat) Francisco-José Vázquez-Polo PhD; Miguel A. Negrín PhD; and Jaime Corral, MD.
Sources of fundingInstituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Madrid, Spain) PI050402, Spanish Respiratory Foundation 2005 (FEPAR) and Air Liquide Spain.
Clinicaltrial.gov identifier: NCT01405976.
Conflict of interestNo conflicts exist for the authors.
We are indebted to Verónica Rodríguez for her assistance in the translation of the manuscript and to Vanessa Iglesias for her technical assistance. Juan F Masa has full access to all data from the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Juan A. Riesco, MD1,21,22; Rocio Gallego, MD1,22; Nicolás González-Mangado, MD, PhD6,21; Teresa Gomez-Garcia, MD6,21; Maria A. Martinez-Martinez, MD7; Elena Ojeda-Castillejo, MD8; Daniel López-Padilla, MD8; Santiago J. Carrizo, MD, PhD, Prof9,21; Begoña Gallego, MD, PhD9; Mercedes Pallero, MD10,21; Odile Romero MD10,21; Maria A. Ramón, PT, MSc10,21; Eva Arias, MD,11,21; Jesús Muñoz-Méndez, MD, PhD11,21; Cristina Senent, MD, PhD12; Jose N. Sancho-Chust, MD, PhD12; Nieves B. Navarro-Soriano, MD13,21; Emilia Barrot, MD, PhD4; José M. Benítez, MD17; Jesús Sanchez-Gómez, MD17; Rafael Golpe, MD, PhD18; María A. Gómez-Mendieta, MD, PhD19; Silvia Gomez, MD2,21; and Mónica Bengoa, MD20.
Centers: (1) Respiratory Department, San Pedro de Alcántara Hospital, Cáceres, Spain; (2) Institut de Recerca Biomédica de LLeida (IRBLLEIDA), Lleida, Spain; (3) Respiratory Department, Virgen del Puerto Hospital, Plasencia, Cáceres, Spain; (4) Unidad Médico-Quirúrgica de Enfermedades Respiratorias, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío, Sevilla, Spain (5) Respiratory Department, University Hospital, Burgos, Spain; (6) Respiratory Department, IIS Fundación Jiménez Díaz, Madrid, Spain; (7) Respiratory Department, Valdecilla Hospital, Santander, Spain; (8) Respiratory Department, Gregorio Marañón Hospital, Madrid, Spain; (9) Respiratory Department, Miguel Servet Hospital, Zaragoza, Spain; (10) Respiratory Department, Vall d’Hebron Hospital, Barcelona, Spain; (11) Respiratory Department, Doce de Octubre Hospital, Madrid, Spain (12) Respiratory Department, San Juan Hospital, Alicante, Spain; (13) Respiratory Department, Alava University Hospital IRB, Vitoria, Spain; (14) Nursing Department, Extremadura University, Cáceres, Spain; (15) Department of Quantitative Methods, Las Palmas de Gran Canaria University Canary Islands, Spain; (16) Medicine/Pulmonary and Critical Care, University of Chicago, IL, USA; (17) Respiratory Department, Virgen de la Macarena Hospital, Sevilla, Spain; (18) Respiratory Department, Lucus Agusti Universitary Hospital, Lugo, Spain; (19) Respiratory Department, La Paz Hospital, Madrid, Spain; (20) Respiratory Department, University Hospital, Las Palmas, Spain; (21) CIBER de enfermedades respiratorias (CIBERES), Madrid, Spain; (22) Instituto Universitario de Investigación Biosanitaria de Extremadura (INUBE).