In a preliminary study, we showed that many COVID-19 pneumonia patients when discharged from the acute care setting respond significantly to inhaled short-acting β2-agonists (SABAs). Such response is observed in patients without an obstructive pattern 1 but also in those experiencing lung interstitial impairment which represents a characteristic feature of COVID-19 patients.2,3
Therefore, we investigated whether the bronchial response to SABAs may serve as functional predictor of lung function improvement induced by pulmonary rehabilitation (PR).
Within 48h of admission to the Pulmonary Rehabilitation Unit of Istituti Clinici Scientifici Maugeri of Telese Terme, Italy, consecutive post-COVID-19 patients discharged from an acute care setting were screened for eligibility (Table 1). Bronchial response to inhaled salbutamol 400μg was assessed according to the American Thoracic Society/European Respiratory Society (ATS/ESR) Task Force4 using automated equipment (Vyasis FlowScreen II Spirometer, Milan, Italy). Then, all included patients underwent a 5-week PR program with daily sessions (6 sessions/week). The program consisted of 30 sessions following the official ATS/ERS statement on PR.5 All exercises were performed at moderate to high intensity to obtain an overload stimulus. Based on dyspnea and fatigue symptom scores, the training intensity was progressively increased. At the end of the RP program, lung functional tests were repeated, including assessment of bronchial response to salbutamol.
Patients’ characteristics.
| Entire groupn=84 | No OLDn=70 | COPDn=12 | Asthmaticn=2 | Ventilatedn=24 | |
|---|---|---|---|---|---|
| Age (years) | 60.0±9.7 | 59.6±9.6 | 64.5±7.6 | 43.5±3.5 | 59.3±8.0 |
| Sex | 12 F | 10 F | 1 F | 1 F | 4 F |
| BMI (kg/m2) | 29.9±5.8 | 29.5±5.6 | 31.7±5.9 | 36.0±12.7 | 30.5±6.4 |
| Smokers (n) | 10 | 5 | 5 | 0 | 3 |
| Ex-smokers (n) | 35 | 28 | 7 | 0 | 11 |
| WHO classification | 3.6±0.5 | 3.7±0.5 | 3.5±0.5 | 4.0±0 | 4.0±0.2 |
| Length of stay for hospitalized patients (days) | 23.2±10.5 | 22.8±10.2 | 23.4±10.9 | 20.0±2.8 | 26.2±8.5 |
| FEV1 (L) | 2.3±0.8 | 2.5±0.8 | 1.7±0.6 | 2.5±0.1 | 2.4±0.9 |
| FEV1% predicted | 76.0±11.0 | 79.4±19.7 | 58.9±17.2 | 79.5±6.4 | 74.3±18.4 |
| FVC (L) | 2.9±1.0 | 3.0±1.0 | 2.5±0.8 | 3.0±0.2 | 2.8±1.1 |
| FVC % predicted | 73.8±19.7 | 75.1±19.9 | 68.3±18.3 | 75.5±0.7 | 68.3±17.8 |
| FEV1/FVC | 82.2±10.4 | 84.3±6.7 | 72.7±15.5 | 86.5±4.9 | 86.0±6.5 |
N: number; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; OLD: obstructive lung disease; COPD: chronic obstructive pulmonary disease; F=females; WHO: world health organization of COVID-19 severity classification.
The study was approved with reference number ICS 11/20 and all patients provided written informed consent.
Data were expressed as mean and 95% confidence interval (CI) (standard errors have been reported in figures). Analysis of spirometric data before and after PR was performed using the Student's t test for paired variables. Furthermore, a Pearson correlation analysis was performed to determine if there were significant correlations between the improvements in FEV1 and FVC induced by salbutamol before and after PR. We used the Chan's interpretation of the r values6 to name the strength of the linear relationship between the two variables. A p<0.05 was considered as being of significance for all tests.
Among the 84 patients in whom reversibility tests could be performed, 14 had concomitant obstructive lung disease (OLD), 12 suffering from chronic obstructive pulmonary disease (COPD) and 2 from asthma (Table 1). Forty-nine and 52 patients, respectively, had FEV1 and FVC values lower than 80% of predicted values.
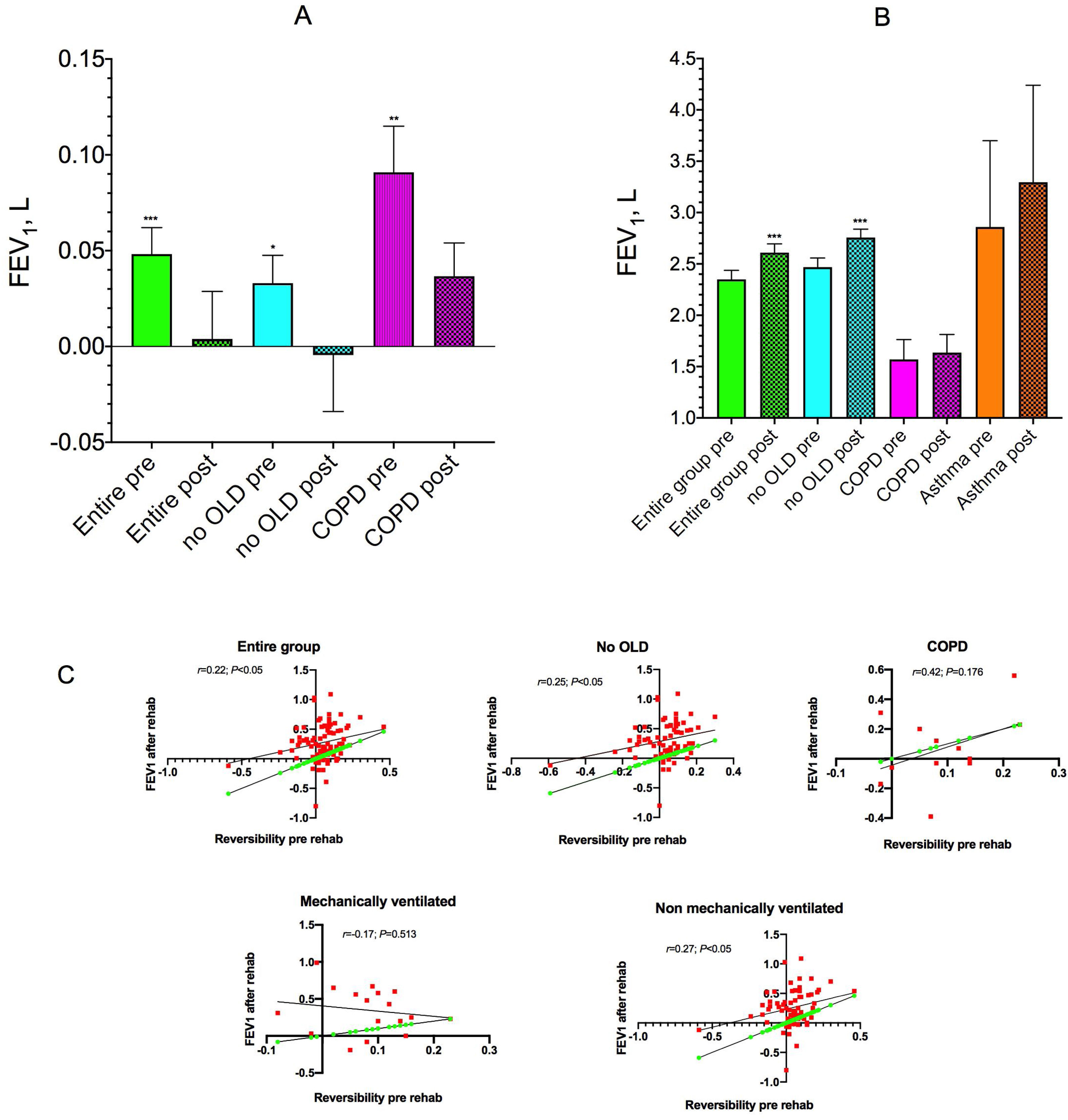
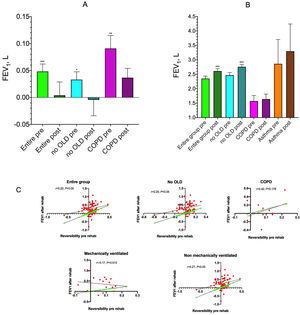
Before PR, mean improvement in FEV1 induced by salbutamol for all patients was 48.0mL (95% CI: 20.4–75.6mL; P<0.001) (Fig. 1A). FEV1 increased by 90.8mL (95% CI: 37.7–144.0mL; P<0.01) and 325.0mL in COPD and asthmatic subjects, respectively, but excluding the subjects with OLD, FEV1 improvement was 32.7mL (95% CI: 3.6–61.9mL; P<0.05).
Mean (SE) increase from baseline of FEV1 after salbutamol 400μg in the entire group, in subjects without concomitant obstructive lung diseases (no OLD), and in COPD patients (A), and mean (SE) pre-salbutamol test in the entire group, in no OLD subjects, in COPD patients and in asthmatics before (pre) and after (post) rehabilitation (B). Correlation between improvements in FEV1 induced by salbutamol before starting the rehabilitation program and the improvements in parameter measured at the end of rehabilitation in different groups (C). *P<0.05, **P<0.01, ***P<0.001 vs pre-salbutamol (A) and vs pre-rehabilitation (B).
After PR, FEV1 increased by 260.5mL (95% CI: 190.2–330.8mL; P<0.001) in the entire population (Fig. 1B), while the number of patients with FEV1 and FVC values below 80% of predicted decreased from 34 to 30 patients. However, FEV1 and FVC values decreased more than 5% predicted in 5 patients. In subjects without OLD, COPD and asthmatics FEV1 increased by 288.6mL (95% CI: 210.12–367.0mL; P<0.001), 67.5mL (95% CI: −87.4–222.4mL; P=0.358), and 435.0mL, respectively.
Changes in FEV1 induced by salbutamol before rehabilitation significantly correlated with the improvement in FEV1 observed after PR in the entire group (r=0.22; P<0.05) and in patients without OLD (r=0.25; P<0.05), but not in COPD patients (r=0.42; P=0.176) (Fig. 1C).
In patients who required mechanical ventilation during hospitalization for COVID-19 pneumonia, the mean salbutamol induced increase in FEV1 was 82.4mL (95% CI: 43.6–121.1mL; P<0.001) and 347.6mL (95% CI: 187.3–508.0mL; P<0.001) before and after rehabilitation, respectively. In patients who did not require mechanical ventilation the mean FEV1 change induced by salbutamol was 39.2mL (95% CI: 6.1–72.4mL; P<0.05) and 238.4mL (95% CI: 159.0–317.7mL; P<0.001) before and after rehabilitation, respectively.
In non-ventilated subjects a significant and direct correlation between salbutamol induced changes in FEV1 before and after rehabilitation (r=0.27; P<0.05) was observed. In contrast, a non-significant correlation was found in patients who had been mechanically ventilated (r=−0.17; P=0.513) (Fig. 1C).
Before rehabilitation, salbutamol improved FVC by an average of 71.8mL (95% CI: 31.7–111.9mL; P<0.001), 57.0mL (95% CI: 15.0–99.0mL; P<0.01), 125.0mL (95% CI: 16.8–266.8mL; P=0.078) and 270.0mL (P=0.226) in the entire population, in patients without OLD, in COPD and asthmatic patients, respectively. The mean value of FVC before salbutamol increased substantially after PR (entire sample: 387.5mL, 95% CI: 298.1–476.9mL, P<0.001; no OLD subjects: 429.0mL, 95% CI: 334.6–523.4mL, P<0.001; COPD patients: 102.5mL, 95% CI: −160.7–365.7mL, P=0.410; asthma patients: 645.0mL).
Only when OLD patients were excluded from the Pearson correlation analysis, the rehabilitation-induced improvement in FVC correlated with salbutamol-induced increase in FVC at admission (r=0.26, P<0.05 vs. r=0.04, P=0.735).
In patients who had been mechanically ventilated, the mean FVC improvement after salbutamol was 129.4mL (95% CI: 50.9–207.9mL, P<0.01) and 13.6mL (95% CI: −43.0–70.1mL, P=0.619) before and after rehabilitation, respectively. In patients who were not mechanically ventilated, these changes were 54.8mL (95% CI: 7.9–101.8mL, P<0.05) and 32.4mL (95% CI: −3.1–67.9mL, P=0.072), respectively.
Our results consistently show that PR in post COVID-19 patients may result in a substantial improvement in lung function, with the normalization of lung functional parameters in a high percentage of cases that are without pre-existing OLD. In fact, 31 of our 84 patients had FEV1 greater than 90% predicted at the end of the PR program and in other 22 subjects, FEV1 was between 80.0 and 89.9% predicted.
In the COPD population, the Pearson correlation coefficient was higher, indicating a fair association between the salbutamol-induced improvements in FEV1 before and after PR. However, the reliability of the linear model also depends on how many observed data-points are in the sample. In our COPD patients, the P value was 0.176 (not statistically significant), which reflected the very small sample size (n=12). It will therefore be essential to conduct a specific study on a larger population of COPD patients.
In the absence of a control group, it is hard to determine how much of the improvement was the consequence of rehabilitation rather than the spontaneous recovery of lung function. Unfortunately, the challenges posed by the pandemic did not allow us to enroll a control group. COVID-19 is in fact an obstacle to the use of the standard diagnostic criterion, namely spirometry, which is considered a procedure that generates aerosols and which can therefore increase the risk that someone who is an asymptomatic carrier of the virus can easily spread it in an outpatient setting.7
The significant PR-induced improvement in lung function in mechanically ventilated patients, none of whom had pre-existing chronic OLD, is noteworthy. The reasons for this improvement are not univocal. The greater severity of the disease, on the one hand, and the effects of mechanical ventilation on the trophism of the respiratory muscles, on the other hand, may have contributed to the observed differences.8
In conclusion, our results suggest that the reversibility test with SABAs may be a predictor of the functional improvements induced by rehabilitation in post COVID-19 patients. Larger controlled studies are needed to confirm our preliminary findings.
Authors’ contributionsM. Maniscalco: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review and editing. S. Fuschillo: Investigation. P. Ambrosino: Data curation, Investigation, Methodology, Writing – review and editing. S.E. D’Anna: Investigation. M.S. Accardo: Investigation. M.G. Matera: Formal analysis, Validation, Writing – review and editing. M. Cazzola: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review and editing. All Authors read and approved the final version of the manuscript.
Funding sourcesNo funding or economic support were received for this study.
Conflicts of interestsThe authors have no conflicts of interests to declare.
The Authors thank Anna Ciullo and Silvia Stufano for their technical assistance.