The radiologic evolution of global structural lung damage in patients with cystic fibrosis (CF) measured with different scoring systems has been published by several authors.1,2 However, there is less information about the temporal evolution of each specific anatomic item of the CT scoring scales. In our analysis we wanted to assess in adults with CF which temporal change of each of the radiological items assessed by Brody II scale was related with exacerbations or the need for oral or intravenous antibiotic administration. Thus, changes in these parameters could indicate the need for a more specific or aggressive treatment for the patients before the irreversible damage appears.
An ambispective longitudinal study was conducted from 2007 to 2018, retrospective phase 2007–2017 and prospective phase 2017–2018, enrolling adult patients from a cystic fibrosis unit. Inclusion criteria were: CF patients over 18 years, with at least two consecutive thoracic routine CT valid for diagnosis, with respiratory function test at the time of CT. Exclusion criteria were: having only one CT, or an acute exacerbation during the 4 weeks prior to the procedure. The institutional ethics review board approved the study. For the selected patients two CT images were anonymized, randomized, blinded to the clinical and spirometric data and scored using Brody II3 scale by one senior radiologist. Subscores were determined evaluating each individual item in the Brody II scale: bronchiectasis, mucous plugging, peribronchial thickening, parenchymal alterations (including dense parenchymal opacity, ground glass opacity, cyst or bullae) and air trapping. For each subscore, the annual change was calculated by subtracting the first score from the last one and dividing by the number of elapsed years between them. Annual FEV1% variation was calculated by subtracting the FEV1% at the last CT time from the FEV1% at the first CT time and dividing by the number of years elapsed between both of them. The annual number of exacerbations and the number of oral or intravenous antibiotic treatments were measured by dividing total number exacerbations or oral or intravenous antibiotic treatments by the number of years between the initial and final CT study. Correlations between the annual score change of each radiological item and the following variables were analyzed: annual FEV1% variation, annual number of exacerbations and number of oral or intravenous antibiotic cycles.
Of the total 83 patients evaluated at the CF clinical unit from 2007 to 2018, 64 (33 males) finally met the inclusion criteria; median age was 26.84 (SD 7.92) years. Demographic, clinical, microbiological and spirometric characteristics of the study cohort are shown in Table 1. The median interval between the two CT studies was 3.88 (SD 1.59) years. The mean spirometric values of the patients showed dynamic lung volumes lower than those of the general population, both in absolute values and as percentages of the reference values. FEV1% was 71.78 (SD 19.39) at the time of the first CT, and 66.74 (SD 19.94) at the last CT. Mean FEV1% annual variation was (−1.30, SD 2.66), indicating decrease of respiratory function. In the last study, score was higher and the annual score change was positive for every item, except for air trapping in which these parameters were lower and negative, respectively. These results indicate worsening of anatomic status over time, except for air trapping. Annual changes of mucous plugging, parenchymal alterations and air trapping were the radiological parameters that correlated with exacerbation (r was 0.291, 0.317 and 0.251, respectively, p value<0.05). Evolution of mucous plugging correlated with FEV1% worsening (r=−0.253, p value <0.05). (Fig. 1 shows the evolution of mucus plugging). No statistically significant relationship was demonstrated between annual changes of bronchiectasis or peribronchial thickening and number of exacerbations, FEV1% variation, or antibiotic cycles. The interobserver and intraobserver agreements for the different items were respectively: for bronchiectasis (0.85 and 0.99), mucus plugging (0.73 and 0.82), peribronchial thickness (0.89 and 0.97), parenchymal alterations (0.80 and 1) and air trapping (0.73 and 0.97), p value<0.05.
Clinical and demographic characteristics of the study population.
| Variables | n=64 |
|---|---|
| Demographics | |
| Age at first CT, years, mean, SD | 26.84±7.92 |
| Sex (male/female) number of patients (%) | 33 (51.64)/31 (48.44) |
| BMI, kg/m2, mean, SD | 22.53±3.03 |
| Genotype, number of patients (%) | |
| Phe508del homozygous | 21 (32.81) |
| Phe508del heterozygous | 26 (40.63) |
| Others | 17 (26.56) |
| Presence of co-morbidities, number of patients (%) | |
| Pancreatic insufficiency | 43 (67.19) |
| Hemoptysis | 10 (15.63) |
| Lung transplantation | 6 (9.38) |
| Exitus | 1 (1.56) |
| CFTR modulator therapies, number of patients (%) | 0 (0) |
| Annual exacerbations mean, SD | 2.88 (2.49) |
| Annual oral antibiotic treatments mean, SD | 2.41 (2.19) |
| Annual intravenous antibiotic treatments mean, SD | 0.33 (0.75) |
| Sputum culture, number of patients (%) | |
| Airway colonization at any time | 63 (98.44) |
| Staphylococcus aureus | 48 (75) |
| Pseudomonas aeruginosa | 37 (57.81) |
| Haemophilus influenzae | 31 (48.44) |
| Burkholderia cepacia | 17 (26.56) |
| MRSA | 15 (23.44) |
| Mycobacterium avium | 10 (15.63) |
| Mycobacterium abscessus | 8 (12.5) |
| Aspergillus sp | 9 (14.06) |
| Spirometric variables, % predicted, mean, SD | |
| FEV1 at first CT time | 71.78 (19.39) |
| FEV1 at last CT time | 66.74 (19.94) |
| FEV1 annual change | −1.30 (2.66) |
CT: computed tomography, SD: standard deviation, BMI: body mass index, MRSA: methicillin resistant Staphylococcus aureus, FEV1: forced expiratory volume in one second, CFTR: cystic fibrosis transmembrane regulator.
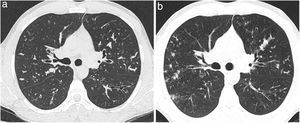
(a, b) Example of the evolution of two consecutive axial CT images (a: CT axial image at the first CT and b: CT axial image at the last CT) of a patient with cystic fibrosis demostrating a progression of mucus plugging. Brody II subscore for mucus plugging at the first CT was 5 and at the last CT was 10. There was also a decline of FEV1% (FEV1% 1 was 79% and FEV1%-2 was 66%). CT: computed tomography. FEV1%-1: forced expiratory volume in 1 second at the first CT time. FEV1%-2: forced expiratory volume in 1 second at the first CT time.
We found that temporal changes of mucus plugging, parenchymal alterations and air trapping showed relation with number of exacerbations, while only progression of mucus plugging showed a relation with FEV1% temporal decline. On the contrary, the evolution of bronchiectasis and peribronchial thickening did not show relation with exacerbations. Temporal evolution of air trapping demonstrated a slight improvement with time. On the other hand, we found that FEV1% annual variation did not correlate with exacerbations or with number of antibiotic treatments. Brody et al.4 demonstrated that the change in CT scores correlated moderately well with the number of exacerbations. The poor correlation between the variation of FEV1% and exacerbations suggested that CT might be superior to functional parameters to predict the clinical evolution of these patients over time. Other studies have reported similar results.1,5–8
Tepper et al.9 described how mucus plugging could be considered a previous stage for the development of established bronchiectasis. The presence of mucus plugging could be a prognostic factor for the development of bronchial enlargement and would justify more exhaustive treatment in patients with occupied bronchi. We found a correlation of mucus plugging with exacerbations and functional respiratory deterioration, we consider that these data are especially relevant since the presence of mucus plugging facilitates colonization and infection leading to a progressive structural damage. Therefore, we propose that increasing mucus plugging with time will correlate with a decline of respiratory function with clinical complications. Recently, Robinson et al.10 found mucus plugging and air trapping as the most sensitive indicators of progressive CF lung disease in children. In our study, the size of bronchiectasis and its extension also increased over time, similar to data previously published by Tepper et al.11 Although bronchiectasis has been considered the primary alteration in CF translating irreversible damage to the parenchyma, in our study evolution of this parameter did not show correlation with exacerbation or with the need for antibiotic treatment. An explanation for this could be that since injuries are irreversible, once they appear progression is slow. To our knowledge this is the first study reporting the correlation of temporal evolution of parenchymal alterations such as dense parenchymal opacities, ground glass opacities, cysts or bullae with exacerbations and with number of oral antibiotic cycles. Contrary to the rest of the anatomical changes, in our study air trapping showed a slight improvement over time. In the pediatric age, air trapping can be one of the first manifestations of CF. More than half of children aged below 6 years diagnosed by newborn screening present air trapping on CT, and many of them are asymptomatic.12 Loeve et al.13 described the reversibility of air trapping. The improvement of air trapping observed in our work might be due to new pharmacological treatments.14 However, it could also be due to the fact that sometimes patients do not perform properly the requested expiration during the acquisition of CT images. We found correlation between air trapping and exacerbations, similar to other reports.10
In conclusion, in adult patients with CF the temporal evolution of mucus plugging, parenchymal alterations and air trapping correlated with risk of exacerbations. Changes in mucus plugging correlated with FEV1% worsening. No statistically significant relationship was found for annual variation of bronchiectasis or peribronchial thickening with exacerbations.
We would like to thank the statistician Maria Teresa Pastor for her collaboration in the statistical analysis of this work.