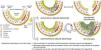
The main presentations of the pulmonary nontuberculous mycobacterial disease are the fibrocavitary, similar to pulmonary tuberculosis, and the nodular bronchiectasis, permanent enlargements of the airways.1 Bronchiectasis has been proposed to arise from a cycle of events characterized by impaired mucociliary clearance and subsequent retention of airway secretions. The consequence is the establishment of chronic infections that promote an inflammatory response that results in injury and pathologycal remodeling of the airways leading to bronchiectasis.2 Bronchiectasis and nontuberculous mycobacteria, particularly M. avium complex and M. abscessus, are now widely accepted to be inextricably linked.3 A hallmark of bronchiectasis is neutrophilic inflammation, with an elevated number of neutrophils in the airway of patients and it has been argued that airways remodeling is the product of neutrophil proteases.4 We have studied whether high concentrations of neutrophils are able to inhibit mycobacterial growth in vitro, as have been reported for Staphylococcus epidermidis.5
Analyzed strains were clinical isolates from patients with the indicated diseases. Mycobacterium abscessus abscessus 12aba (diabetes type II, monoclonal gammopathy, previous tuberculosis) and 239aba (Kartagener syndrome) and Mycobacterium mageritense 110mg (chronic obstructive pulmonary disease). Colonies from both M. abscessus strains exhibited the smooth morphology in 7H11 agar. Neutrophils were isolated from peripheral blood by two consecutive Polymorphprep (Serumwerk Bernburg AG) density gradient sedimentation and leukocytes by dextran gradient sedimentation and, to remove erythrocytes, a subsequent Polymorphprep step. Infected neutrophils in serum free X-VIVO 15 medium (Lonza) were lysed by ultrasonication and decimal dilutions were incubated in 7H9 medium in 96-well plates at 37°C for 2–3 days. Colony-forming units (CFU) were counted under an inverted DMIL phase microscope (Leica).6
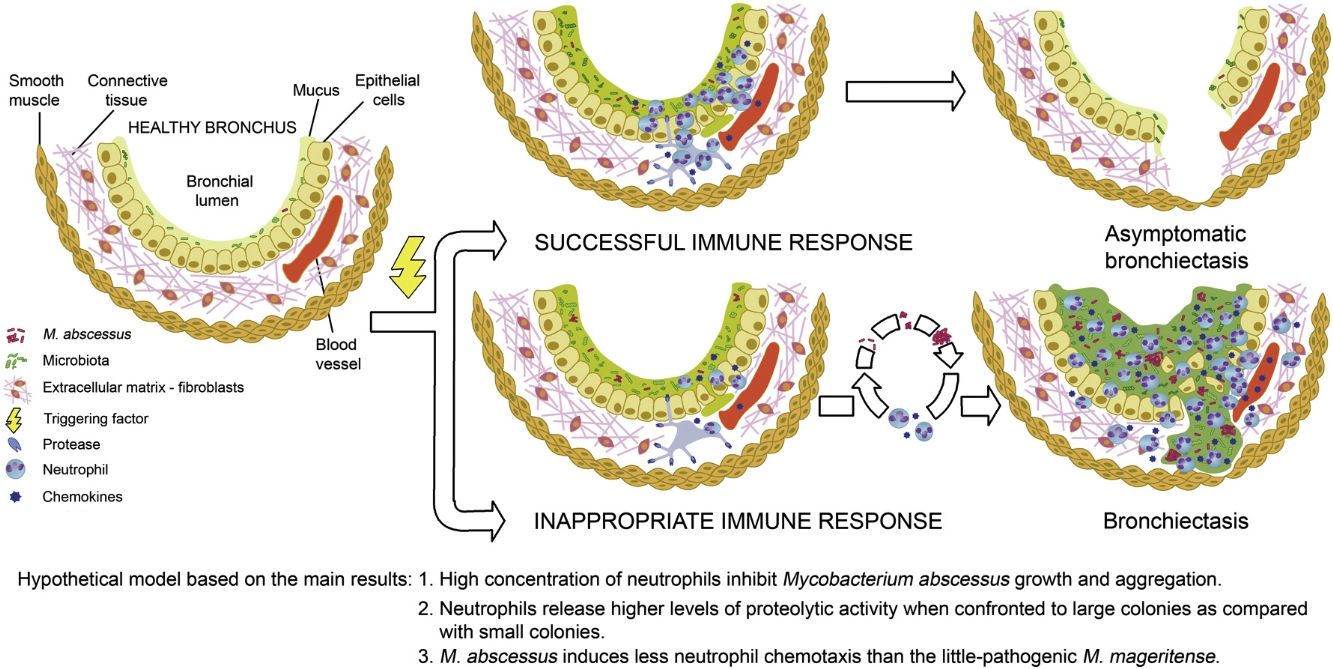
Mycobacteria inoculated in medium was used as reference of growth in the absence of neutrophils. Non-significant differences of M. abscessus multiplication were observed after 1 day of infection (Fig. 1A). On the contrary, after 2 and 3 days there was a significantly lower multiplication at the highest concentration of neutrophils (2 and 3×105neutrophils). Although the number of recovered bacteria was always larger than the inoculum, it may not be ruled out an initial bactericidal activity that is not detected because surviving bacteria multiply rapidly. Similar results were obtained with two additional M. abscessus strains, and two M. mageritense strains, a mycobacterium not associated in the scientific literature to bronchiectasis (data not shown).
(A) Influence of neutrophil concentration on M. abscessus growth. Infection of neutrophils with 103 mycobacteria (strain 239aba) in 100μl (96-well plates). Inoculation of mycobacteria in X-VIVO 15 medium (“No neutrophils”) served as growth controls (n=12) of multiplication in the presence of increasing concentrations of neutrophils (n=6). Infections were lysed at 1, 2 and 3 days and colony forming units were enumerated. Statistical analysis of groups within each day of lysis was performed by one-way ANOVA. Pairwise comparisons were analyzed by Dunnet's post hoc test, comparing each concentration of neutrophils with the control (“No neutrophils”). NS, not significant. P<0.05 was considered significant. (B) Effect of replenishment of in vitro infections with fresh neutrophils. 3×105 neutrophils were infected with 103 mycobacteria (strain 239aba). Indicated wells were replenished with 3×105 neutrophils recently purified after 1 or 2 days. Statistical analysis of groups within each day of lysis was performed by Mann–Whitney's test (day 2) or Kruskal–Wallis test (day 3 and 4). Pairwise comparisons with the “No addition” control were analyzed by Dunn's post hoc test. Results are presented as median and 95% confidence interval of log10 transformed colony forming units (n=6). NS, not significant. P<0.05 was considered significant. (C) Proteolytic activity in supernatants of neutrophils infected with M. abscessus. 3×103 bacteria were grown for 5 days, 30h or 0h, or the corresponding number of isolated bacteria (106 bacteria at 30h or 7×107 bacteria at 5 days) in 300μl (48-well plates), before 6×105 neutrophils in 200μl were added to each well, incubated for 24h and supernatants removed. Gelatinase activity in supernatants was measured as the proportion of undigested gelatin that remained attached to streptavidin coated wells. Tukey's test was used for post hoc analysis of the one-way ANOVA analysis. Results are presented as mean and standard deviation (n=6). NS, not significant. P<0.05 was considered significant. (D) Neutrophil chemotaxis. 103 bacteria were seeded in 450μl of medium (48-wells plates) and allowed to grow for 5 days. The day of the experiment, an additional well was inoculated with 103 bacteria and 105 leukocytes from 6 healthy donors, in 50μl of medium, were immediately added to all wells and incubated for 24h. Each supernatant (235μl) was inoculated in two wells of HTS Transwell-96 well permeable supports, 8.0μm pore size (Corning). Neutrophils (105 in 75μl) were isolated from two healthy donors and inoculated in the inserts above each of the two wells with the same supernatant. After 2h inserts were removed and cells were enumerated using a Neubauer chamber. Number of neutrophils were normalized to cells migrated in supernatants from leukocytes that had not been infected. Results are presented as median and 95% confidence intervals (n=6×2). Comparison between 0h and 5 days was performed by the Mann–Whitney test (empty lines). Comparison of the three strains at 5 days was performed by the Kruskal–Wallis test (dark lines), and Dunn's test was used for post hoc analysis. NS, not significant. P<0.05 was considered significant.
To simulate the arrival of new neutrophils at the site of infection, additional fresh neutrophils were added to wells after 1 or 2 days (Fig. 1B). It was observed a lower level of M. abscessus multiplication when cells were lysed on day 2 or 3 if the first day fresh neutrophils were added. When they were added on the second day no growth inhibition could be detected at day 3. At day 4 mycobacterial growth became similar in all cases, suggesting that a continual arrival of fresh neutrophils may be required to inhibit multiplication. A comparable observation has been reported for Staphylococcus aureus because given time enough to multiply and aggregate, it becomes recalcitrant to clearance by neutrophils.7
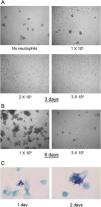
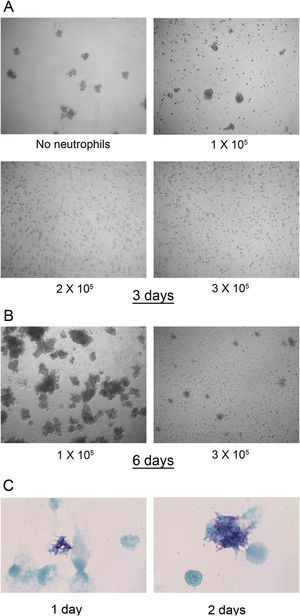
In fresh cultures no differences were found in the number of M. abscessus colonies that were visible after 3 days of infection in wells with 0–1×105 neutrophils. However, at 2 and 3×105 neutrophils, colonies were not detected (Fig. 2A). This inhibition seems to depend on live neutrophils. We have observed that neutrophil lysates, both M. abscessus infected or not infected, inhibits mycobacterial growth in X-VIVO 15 medium (data not shown). After 6 days large colonies began to aggregate. Colonies also developed in wells with 3×105 neutrophils but in lower numbers and smaller (Fig. 2B). The formation of visible colonies did not depend on the absolute number of mycobacteria. The mean log10 CFU (Fig. 1A) in wells with 1×105 neutrophils after two days was similar to that in wells with 2×105 neutrophils after three days (6.33 SD 0.48 vs 6.77 SD 0.67), but colonies were visible only in the former. Although colonies were not observed for the first two days in 3×105 neutrophils, mycobacteria were multiplying, as revealed by the Kinyoun stain, with small microcolonies formed after 1 day that grew larger the second day (Fig. 2C).
M. abscessus 239aba colony formation in different concentrations of neutrophils. Infected neutrophils were left undisturbed for 3 days (A) or 6 days (B) and microphotographs were taken at ×100 magnification. (C) Kinyoun-Brilliant Green staining of infected neutrophils (3×105) for 1 or 2 days at ×1000 magnification.
Current hypotheses indicate that neutrophil proteases are responsible for airway remodeling in mycobacterial infection.4 To investigate the protease activities released by neutrophils when confronted to M. abscessus colonies, we performed a gelatinase assay with biotinylated gelatin as previously described8 (Fig. 1C). In supernatants with high proteolytic activities fewer biotin groups are available and a lower signal is developed when using streptavidin-horseradish peroxidase (Becton Dickinson Biosciences) and TMB (tetramethylbenzidine) ELISA peroxidase substrate (Rockland). It was observed that there were no statistical differences in the proportion of undigested gelatin when neutrophils were confronted with isolated mycobacteria equal to the inoculum (103) or with colonies grown for 30h (Fig. 1C). In contrast, 5 days colonies prompted the release of a higher proteolytic activity, indicating that it was dose-dependent. No statistical differences were found between bacteria forming colonies and isolated at each of these time points. Aggregation is viewed as a form of biofilm in Pseudomonas aeruginosa, and aggregated bacteria are detected in lung biopsies and sputum from cystic fibrosis patients, usually surrounded by neutrophils.9 Furthermore, pulmonary chronic infections by M. abscessus are associated to biofilm development in the lungs.10
Finally, we studied the chemotaxis induced by supernatants from leukocytes, that include all blood cell populations that release chemokines, confronted with isolated mycobacteria (inoculum, 0h) or with large colonies (5 days). M. abscessus strains promoted a similar chemotaxis at both time points. In contrast, M. mageritense incubated for 5 days induced a significantly higher level of chemotaxis than M. abscessus strains. The higher level of chemotaxis induced by M. mageritense may allow in the lungs the initial arrival of enough neutrophils, perhaps explaining why some mycobacteria are unfrequently associated to bronchiectasis.
This study presents several limitations. The described in vitro model is very simple and other factors such as humoral components or other cell types were not analyzed. Although we have used clinical strains, we do not rule out that these results may not be extended to all M. abscessus strains. Furthermore, the source of cells were healthy volunteers, but the results obtained may provide useful clues about the means that M. abscessus use to overcome the lung defenses. The reported findings have not been documented before probably because most models have shorter incubation times, usually hours, and larger multiplicity of infection (1–10 bacteria per cell).11
The higher proteolytic activity released by neutrophils engaged to large colonies is in line with the vortex hypothesis of bronchiectasis development,2 originally presented by Cole as a vicious circle 12. If neutrophils control mycobacterial growth in the initial stages, the formation of large aggregates will be prevented. It is possible that bronchiectasis detected in elders without clinical symptoms13 correspond to old lesions that remain after such a successful neutrophilic immune response.
We speculate that these findings may be clinically relevant because neutrophil immunomodulation of mycobacterial growth and biofilm formation might be an advantageous alternative to eradication with antibiotics.
FundingThis research was supported by Consejería de Sanidad de la Junta de Castilla y León. Gonzalez-Cortés was supported by a grant from Ministerio de Economía y Competitividad, subprogram of technical support staff.
Conflict of interestNone declared.
We thank Dr. Santiago Vivas-Alegre for helpful discussions.