In a clinical phenotype-based management strategy for COPD, it would be preferable to at least assign all patients to a phenotype, but to a single phenotype only. The aim of this study was to evaluate whether all patients are assigned to one and only one phenotype using the Spanish COPD guidelines (GesEPOC) 2017 and to evaluate the criteria that define these categories.
MethodThe Time-based Register and Analysis of COPD Endpoints study (TRACE; clinicaltrials.gov NCT03485690) is a prospective cohort of COPD patients attending annual visits since 2012, which collects GesEPOC phenotypes. Although the GesEPOC recommends that patients considered to be at low risk are not phenotyped, an analysis of the criteria for identifying high- and low-risk phenotypes was performed, comparing the distribution of phenotypes and the criteria applied between these 2 groups.
ResultsThe cohort included 970 patients with a confirmed diagnosis of COPD, divided into 427 (44.02%) low-risk and 543 (55.9%) high-risk patients. The most frequent phenotype was the non-exacerbator (44.9% of high-risk patients). Overall, 20.6% of low-risk patients met criteria for asthma-COPD overlap syndrome, while 9.2% of the cohort did not meet the diagnostic criteria for any phenotype, and 19.1% met the criteria for 2 phenotypes, with no differences between risk groups.
ConclusionsOur data highlight some of the weaknesses of the current clinical phenotype strategy, revealing overlapping categories in some cases, and patients to whom no phenotype was assigned.
En una estrategia de manejo de la enfermedad pulmonar obstructiva crónica (EPOC) basada en fenotipos clínicos sería deseable que todos los pacientes pudieran adscribirse al menos a un fenotipo sin adscribirse a otro. El objetivo de este trabajo fue evaluar si todos los pacientes tienen un fenotipo y sólo uno asignado según la actual guía española de la EPOC (GesEPOC 2017) y evaluar los criterios que los definen.
MétodoEl estudio Time-based Register and Analysis of COPD Endpoints (TRACE; clinicaltrials.gov NCT03485690) es una cohorte prospectiva de pacientes con EPOC en visitas anuales desde 2012 que recoge los fenotipos GesEPOC. A pesar de que GesEPOC recomienda no fenotipar a los pacientes considerados de bajo riesgo, se realizó un análisis de los criterios de identifican los fenotipos en alto y bajo riesgo, comparando la distribución de los fenotipos y sus criterios en estos dos grupos.
ResultadosLa cohorte incluye 970 pacientes con diagnóstico confirmado de EPOC, divididos en 427 (44,02%) pacientes de bajo riesgo y 543 (55,9%) de alto riesgo. El fenotipo más frecuente fue el no agudizador (44,9% de los pacientes de alto riesgo). Un 20,6% de los pacientes de bajo riesgo cumplían criterios de solapamiento entre EPOC y asma. Un 9,2% de la cohorte no cumplía los criterios diagnósticos de ningún fenotipo y el 19,1% cumplía los criterios de dos fenotipos, sin diferencias entre grupos de riesgo.
ConclusionesNuestros datos ponen de manifiesto algunas de las debilidades de la actual estrategia basada en fenotipos clínicos, existiendo solapamiento en algunos casos y pacientes sin fenotipos.
In recent decades, advances in the understanding of the pathogenesis of chronic obstructive pulmonary disease (COPD), better characterization of patients, and the availability of new therapeutic options have considerably reshaped the management of this disease. Consequently, recommendations from guidelines on the diagnosis and treatment of COPD have been undergoing changes, reflecting efforts to move towards a more personalized approach to its clinical management. The two gold standards in the management of COPD in Spain, the Global Initiative for Objective Lung Disease (GOLD) and the Spanish COPD Guidelines (GesEPOC), take 2 different approaches, both of which focus on personalized medicine1,2. Specifically, GesEPOC established a clinical phenotype treatment algorithm when it was first published in 20123, while a major update in 20171 introduced the concept of initially classifying patients by risk, before establishing their clinical phenotype. The introduction of the clinical phenotype concept, first used in COPD in 20104, marked a considerable shift in disease management, permitting an approach that is both intuitive for the clinician and close to the reality of patients. As a result, it has been widely implemented in Spain5 and also adopted in other countries6.
However, a factor that is not explicitly stated in the GesEPOC, but implicitly suggested in the concept of phenotype, is that it would be desirable if, in a phenotype-based model, all patients at least could be assigned to one specific phenotype. Furthermore, because of the implications of the different therapeutic strategies for each phenotype, it would be equally desirable for each patient to be assigned to one single phenotype. So far, however, neither of these conditions has been studied in a cohort of COPD patients. The Time-based Register and Analysis of COPD Endpoints (TRACE) study is a prospective follow-up in a real-life cohort that, among other variables, records the phenotypes proposed by GesEPOC, as well as many of the individual components7. As such, the TRACE cohort could provide a more detailed evaluation of GesEPOC phenotypes. The aim of this study was to analyze the clinical phenotypes of COPD patients in the TRACE cohort in order to evaluate their distribution and to study the criteria on which they are based.
MethodTRACE methodology has been published elsewhere7. Briefly, TRACE (clinicaltrials.gov NCT03485690) is a prospective observational study of single-center non-interventional cohorts. The sample comprises only patients with COPD, identified in face-to-face visits according to the current diagnostic criteria1. The protocol does not prespecify any exclusion criteria except for complete reversibility of lung function tests during follow-up.
Patient inclusion began in January 2012. After the cases are identified, patients are prospectively followed indefinitely in annual visits until they die or are lost to follow-up. All patients receive their prescribed medications and therapeutic interventions throughout the study, implementing any medication changes ordered by their treating physician in response to their clinical status. During annual visits, clinical, functional, radiological and laboratory information is recorded using a standardized questionnaire for all visits. The primary clinical outcome is survival. Secondary objectives include dyspnea measured by the mMRC scale, number of moderate or severe exacerbations defined according to GesEPOC, and respiratory function.
The variables collected are: sociodemographic (gender, age), history of smoking, comorbidities, stable phase clinical status during the previous year (including evaluation of dyspnea, cough and sputum production, sputum color if present, and self-reported wheezing), exacerbations and hospitalizations in the previous year, current pharmacological and non-pharmacological treatment, and complementary tests, including at least standard chest X-ray, pre- and post-bronchodilator spirometry, and laboratory results (blood eosinophils, alpha-1 antitrypsin, C-reactive protein, and total IgE). Chronic cough and expectoration identified patients with chronic bronchitis with the time frame currently accepted in GesEPOC 20171. For all other clinical criteria, GesEPOC 2017 definitions were adopted1.
This analysis focuses on the evaluation of clinical phenotypes according to GesEPOC 20171. Although GesEPOC recommends that patients considered to be at low risk should not be phenotyped, this study determined the criteria for identifying phenotypes regardless of whether patients were high- or low-risk. The same criteria proposed by GesEPOC 2017 were used for the identification of acute and non-exacerbator phenotypes. For the asthma-COPD overlap (ACO) phenotype, both criteria proposed by GesEPOC (eosinophils >300 cells/μL or bronchodilator reversibility ≥15% and 400 mL) were used, and cases that met 1 or both criteria were recorded8. Because the TRACE cohort study did not systematically perform high-resolution computed tomography or determine lung volumes or diffusion capacity in all cases, the diagnosis of exacerbator phenotype with emphysema was assigned to frequent exacerbators with a mixed pattern on spirometry (an obstructive and restrictive pattern) together with a body mass index (BMI) <25 kg/m2 or frequent exacerbators with a purely obstructive spirometry pattern and a BMI < 21 kg/m2.
EthicsThe protocol was approved by the Biomedical Research Ethics Portal of Andalusia (approval actas 08/2015 and 07/2017). No personal data that could be used to identify patients were collected during the study. The data obtained are kept strictly confidential (Organic Law 3/2018 dated December 5 on Personal Data Protection and Guarantee of Digital Rights, LOPDGDD) and are accessible only by the principal investigator of the project. Cases are anonymized and coded to ensure data confidentiality. Due to the non-interventionist nature of the study, informed consent was waived as no diagnostic tests or therapeutic interventions are carried out. The ethics committee was notified of this fact, which is clearly reflected in the protocol, and approved the procedure.
Statistical planData analysis was performed using IBM SPSS Statistics software (IBM Corporation, Armonk, New York), version 26. For the description of the variables, centrality and dispersion estimators adapted to the nature of each variable and its distribution were used. The distribution of phenotypes and component variables were studied by comparing high- and low-risk patients. Differences between these groups were analyzed using the Student’s t-test for independent data (after applying Levene’s test for equality of variances) or the X2 test (or Fisher's exact test if applicable). The threshold for statistical significance was set at 0.05.
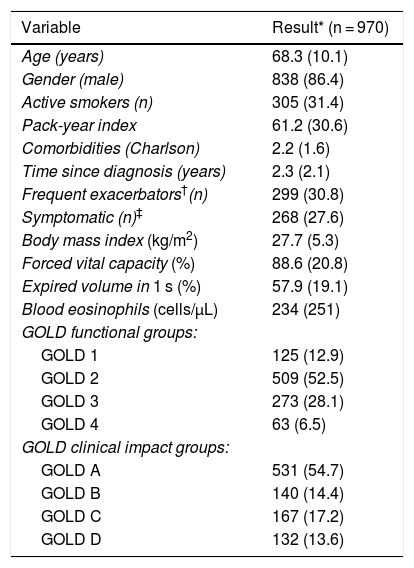
ResultsThis analysis was conducted on 970 patients from the TRACE cohort with a confirmed diagnosis of COPD. The descriptive data of the sample are listed in Table 1. This is a cohort of patients, mostly men, in the seventh decade of life on average, 33% of whom were active smokers. All GOLD clinical and functional groups were represented.
Description of study patients.
| Variable | Result* (n = 970) |
|---|---|
| Age (years) | 68.3 (10.1) |
| Gender (male) | 838 (86.4) |
| Active smokers (n) | 305 (31.4) |
| Pack-year index | 61.2 (30.6) |
| Comorbidities (Charlson) | 2.2 (1.6) |
| Time since diagnosis (years) | 2.3 (2.1) |
| Frequent exacerbators†(n) | 299 (30.8) |
| Symptomatic (n)‡ | 268 (27.6) |
| Body mass index (kg/m2) | 27.7 (5.3) |
| Forced vital capacity (%) | 88.6 (20.8) |
| Expired volume in 1 s (%) | 57.9 (19.1) |
| Blood eosinophils (cells/μL) | 234 (251) |
| GOLD functional groups: | |
| GOLD 1 | 125 (12.9) |
| GOLD 2 | 509 (52.5) |
| GOLD 3 | 273 (28.1) |
| GOLD 4 | 63 (6.5) |
| GOLD clinical impact groups: | |
| GOLD A | 531 (54.7) |
| GOLD B | 140 (14.4) |
| GOLD C | 167 (17.2) |
| GOLD D | 132 (13.6) |
GOLD: Global Initiative for Obstructive Lung Disease; MRC: Medical Research Council.
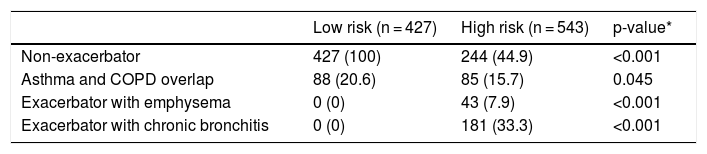
The sample was divided according to GesEPOC risk groups into 427 (44.02%) low-risk and 543 (55.9%) high-risk patients. The distribution of phenotypes according to risk groups is shown in Table 2. Due to the overlap in phenotypes described below, the total number of phenotypes exceeds the number of cases per group. By definition, low-risk patients were non-exacerbators, but 20.6% met the criteria for ACO. The number of ACO patients was significantly higher in the low-risk group.
Distribution of phenotypes by risk groups.
| Low risk (n = 427) | High risk (n = 543) | p-value* | |
|---|---|---|---|
| Non-exacerbator | 427 (100) | 244 (44.9) | <0.001 |
| Asthma and COPD overlap | 88 (20.6) | 85 (15.7) | 0.045 |
| Exacerbator with emphysema | 0 (0) | 43 (7.9) | <0.001 |
| Exacerbator with chronic bronchitis | 0 (0) | 181 (33.3) | <0.001 |
Values expressed as absolute (relative) frequencies of the total number of patients in each risk group.
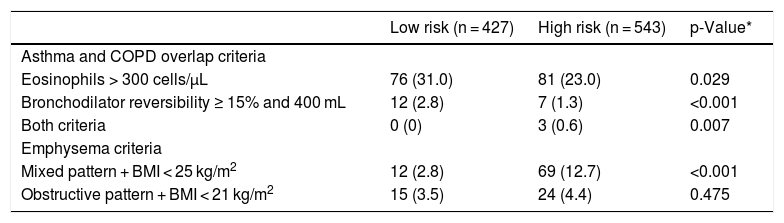
A more detailed analysis of the specific criteria for ACO and exacerbator phenotype with emphysema is presented in Table 3. Most patients with ACO phenotype did not share the criteria for blood eosinophils and bronchodilator reversibility according to the GesEPOC 2017 cut-off points. Significantly more low-risk patients had increased eosinophils than high-risk patients, as well as significantly more frequent bronchodilator reversibility. More patients in the high-risk group met the criterion of mixed pattern with BMI < 25 kg/m2.
Description of the frequency of the components each of the phenotypes.
| Low risk (n = 427) | High risk (n = 543) | p-Value* | |
|---|---|---|---|
| Asthma and COPD overlap criteria | |||
| Eosinophils > 300 cells/μL | 76 (31.0) | 81 (23.0) | 0.029 |
| Bronchodilator reversibility ≥ 15% and 400 mL | 12 (2.8) | 7 (1.3) | <0.001 |
| Both criteria | 0 (0) | 3 (0.6) | 0.007 |
| Emphysema criteria | |||
| Mixed pattern + BMI < 25 kg/m2 | 12 (2.8) | 69 (12.7) | <0.001 |
| Obstructive pattern + BMI < 21 kg/m2 | 15 (3.5) | 24 (4.4) | 0.475 |
Values expressed as absolute (relative) frequencies of the total number of patients in each risk group. BMI: body mass index.
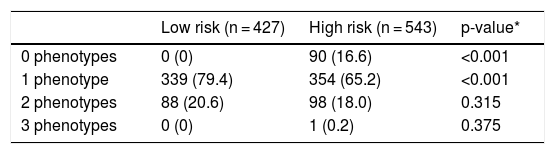
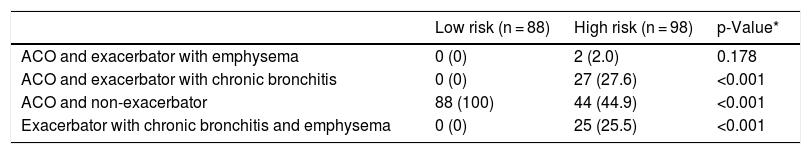
Overlap between phenotypes is shown in Table 4. No defining criteria for any of the phenotypes were identified in 90 (9.2%) cases. These patients were frequent exacerbators who did not have chronic bronchitis and did not meet the emphysema criteria set out in this protocol. Most cases had a single phenotype with a significantly higher frequency among the low-risk group. Two phenotypes were identified in 186 (19.1%) patients, and 1 met the criteria for 3 phenotypes (all but the non-exacerbator). The distribution of patients with double phenotypes is summarized in Table 5. The most common combination was ACO plus non-exacerbator phenotype followed by ACO plus exacerbator with chronic bronchitis phenotype and the 2 exacerbator phenotypes.
Number of phenotypes by risk groups.
| Low risk (n = 427) | High risk (n = 543) | p-value* | |
|---|---|---|---|
| 0 phenotypes | 0 (0) | 90 (16.6) | <0.001 |
| 1 phenotype | 339 (79.4) | 354 (65.2) | <0.001 |
| 2 phenotypes | 88 (20.6) | 98 (18.0) | 0.315 |
| 3 phenotypes | 0 (0) | 1 (0.2) | 0.375 |
Values expressed as absolute (relative) frequencies of the total number of patients in each risk group.
Distribution of patients with double phenotypes.
| Low risk (n = 88) | High risk (n = 98) | p-Value* | |
|---|---|---|---|
| ACO and exacerbator with emphysema | 0 (0) | 2 (2.0) | 0.178 |
| ACO and exacerbator with chronic bronchitis | 0 (0) | 27 (27.6) | <0.001 |
| ACO and non-exacerbator | 88 (100) | 44 (44.9) | <0.001 |
| Exacerbator with chronic bronchitis and emphysema | 0 (0) | 25 (25.5) | <0.001 |
Values expressed as absolute (relative) frequencies of the total number of patients in each risk group. ACO: Asthma and COPD Overlap.
This study shows the distribution of COPD phenotypes by dividing the results into high- and low-risk patients, as defined by GesEPOC 20171. Our results indicate that (1) low-risk patients may have an ACO phenotype; (2) some patients do not fit into any clinical phenotype; and (3) some patients meet the criteria for more than 1 phenotype.
The introduction of clinical phenotypes as a basis for COPD management was proposed by a group of international investigators in 20104 and finally implemented by GesEPOC in 20123. Since then, this approach has spread across Spain and other countries as a way of providing more patient-centered medicine. However, compliance with the criteria and phenotype identification in a large cohort of patients had not been evaluated to date. Our results suggest that the definition of these phenotypes should be further refined to ensure that all patients conform to a phenotype and can be assigned to one and only one phenotype. The consequences of making an erroneous assignation are obvious, as patients would receive treatments that are not optimal for their disease.
Some methodological considerations must be borne in mind if these results are to be interpreted correctly. Firstly, the sample was selected from a specialist COPD outpatient unit, which constitutes a selection bias. Although the data include patients with all levels of functional and clinical involvement, a study in Primary Care may give a different outlook. Secondly, although the cut-off points to identify a patient as emphysematous specified in GesEPOC 2017 are not exact1, the guidelines do emphasize the need to identify emphysema with the use of functional tests or high-resolution computed tomography. In our case, because we do not have this information, we had to estimate which patients were potentially emphysematous. To this end, we selected patients with a restrictive component on spirometry who were not obese and those with a marked decrease in BMI. Although this strategy is not the one recommended by GesEPOC, it is probably a good approach to this phenotype. Identification of the emphysematous patient is clear in specific cases, but this task is more complicated in most COPD patients since most have a clinical presentation that combines airway involvement and parenchyma involvement in the form of emphysema9. The idea of GesEPOC 2017 is to identify predominantly emphysematous patients by clinical/radiological/functional findings. This phenotype is also associated with reduced BMI. In fact, given the close relationship between the pure emphysema phenotype and loss of muscle mass, a decrease in BMI would be a good marker to help identify patients who are very emphysematous or who have predominantly parenchymal involvement10. For this reason, in the absence of a better way of identifying these patients, we rely on BMI and the presence of a restrictive component not explained by obesity as indirect markers of this phenotype. Our data, therefore, could have overlooked patients who, with a more comprehensive study, would have been assigned to the emphysema phenotype; however, even if our selection had been more conservative, our results would have been similar. Therefore, it might be expected that, if patients with emphysema were more accurately identified, there would be a greater overlap of phenotypes, thus supporting our findings. Thirdly, GesEPOC 2017 recognizes ACO in 2 circumstances: when the criteria specified in our cohort are met and when COPD and asthma coexist as 2 diagnoses in the same patient. TRACE is a cohort of COPD patients without asthma. It is possible that in a cohort that includes a more comprehensive patient population with both diagnoses, the ACO criteria would probably be distributed differently and there would probably be a greater overlap between phenotypes than that shown in our results.
Although GesEPOC 2017 recommends that low-risk patients should not be phenotyped1, these patients also have clinical phenotypes. It seems logical to expect low-risk patients to be non-exacerbators, as one of the criteria for the concept of low risk is the number of exacerbations and hospitalizations. However, our data indicate that low-risk patients may have an ACO phenotype according to GesEPOC 2017’s own criteria. This clinical circumstance is to be expected. If patients with ACO respond well to inhaled corticosteroids, they would remain free of symptoms and exacerbations and would have better lung function, so they can continue to be at low risk11. However, contrary to the recommendations of GesEPOC 2017, these patients should not stop receiving inhaled corticosteroids12. Some authors describe these patients as well-controlled. Although the concept of control in COPD was proposed a few years ago13, it seems to identify accurately those patients with low disease impact that is maintained over time with treatment. It would therefore be possible to rethink the concept of high and low risk, and see it more as good or poor control that may change over time, rather a classification of patients with a view to their treatment strategy.
Another factor detected in this study is the identification of patients who do not meet any of the criteria for the phenotypes proposed by GesEPOC 2017. Although in our cohort most patients were assigned to a phenotype, as many as 9.2% of patients in the overall cohort and 16.6% of high-risk patients remained unassigned. These cases were exacerbators who did not meet the criteria for chronic bronchitis, emphysema or ACO. The COPD History Assessment in Spain (CHAIN) study identifies 2.3% patients in this situation14, suggesting that there is room for improvement in the identification of these phenotypes. Although chronic bronchitis is associated with frequent exacerbations15, not all exacerbators have chronic cough and expectoration. Using other more appropriate emphysema criteria may reduce the number of patients without a phenotype, but patients who develop exacerbations without chronic bronchitis or emphysema would still exist, as GesEPOC 2017 suggests.
One finding worth discussing is the existence of a single patient with more than one phenotype. Although most were assigned to one phenotype, about 20% met the criteria for more than one and, anecdotally, 1 patient met the criteria for 3 phenotypes (all except non-exacerbator). Interestingly, GesEPOC 2017 recognizes that there may be difficult-to-classify cases that share characteristics typical of more than 1 phenotype. In these cases, GesEPOC 2017 recommends treating the patient’s most important problem1. This is revealing and raises doubts in routine clinical practice, because the attending physician can assign the patient to one or another phenotype at their discretion, and this has direct consequences on the choice of treatment. The combination of ACO and non-exacerbator in a low-risk patient has already been discussed and is likely to be more related to good disease control than to a clinical phenotype. However, the relationship between ACO and exacerbator with acute bronchitis is interesting. Patients with asthma may also present clinical criteria for chronic bronchitis16 and be exacerbators if they do not receive optimal treatment, so this association should not be an unusual finding. Frequent exacerbators with chronic bronchitis are known to have a different clinical impact than patients with ACO17, but the clinical impact of both phenotypes in tandem has not been explored. The relationship between asthma and emphysema has been poorly studied, but it has been described in the literature and is currently under investigation18. Finally, patients who present clinical criteria for chronic bronchitis and evidence of emphysema are also often identified. The classic phenotypes of chronic bronchitis and emphysema19 represent opposing ends of the COPD spectrum, while the clinical reality shows us that most patients have a component of parenchymal involvement and another component of varying grades of airway involvement20, so it can only be expected that some patients present criteria for both phenotypes.
In conclusion, the introduction of clinical phenotypes has driven the implementation of a more personalized approach to medicine that is more focused on the needs of patients and more intuitive for the clinician. However, our data highlight some of the weaknesses of the phenotype strategy. Consequently, even though clinical phenotypes are a good starting point, it is probably time to move on to a different approach to the disease that maintains the spirit of patient-centered medicine in a way that is intuitive to the clinician and feasible given the limited resources and time constraints of routine practice. A clinical approach based on treatable traits was recently proposed21, and initiatives that include most of these traits in a single proposal are beginning to emerge22. We do not know if in the future we will focus on improving clinical phenotypes, if we will switch to strategies based on treatable traits, or if we will adopt another approach, but we can be sure that our path towards personalized medicine in COPD will be strewn with challenges that can only be resolved by further studies.
FundingThe TRACE project (clinicaltrials.gov NCT03485690) has been funded by an unconditional grant from Laboratorios Gebro Pharma (Barcelona, Spain).
Conflict of interestsJose Luis Lopez-Campos has received honoraria in the past 3 years for lectures, scientific consultancy, clinical trial participation, and preparation of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, and Rovi.
Please cite this article as: Carrasco Hernández L, Caballero Eraso C, Ruiz-Duque B, Abad Arranz M, Márquez Martín E, Calero Acuña C, et al. Deconstruyendo los fenotipos en la EPOC: un análisis de la cohorte TRACE. Arch Bronconeumol. 2022;58:30–4.