Real-time endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is one of the major landmarks in the history of bronchoscopy. In the 10 years since it was introduced, a vast body of literature on the procedure and its results support the use of this technique in the study of various mediastinal and pulmonary lesions.
This article is a comprehensive, exhaustive review of all the available scientific evidence on the general indications for this technique. Results of specific studies on efficacy, safety and cost-effectiveness available to date are examined.
The analysis shows that EBUS-TBNA is a safe, cost-effective technique with a high grade of evidence that is a valuable tool in the diagnosis and mediastinal staging of patients with suspected or confirmed lung cancer. However, more studies are needed to guide decision-making in the case of a negative result. Evidence on the role of EBUS-TBNA in the diagnosis of sarcoidosis and extrathoracic malignancies is also high, but much lower when used in the study of tuberculosis, lymphoma and for the re-staging of lung cancer after neoadjuvant chemotherapy. Nevertheless, due to its good safety record and lack of invasiveness compared to surgical techniques, the grade of evidence for recommending EBUS-TBNA as the initial diagnostic test in patients with these diseases is very high in most cases.
La real-time EndobroBronchial UltraSound-guided TransBronchial Needle Aspiration (EBUS-TBNA) ha supuesto uno de los principales hitos en la historia de la broncoscopia. En los primeros 10años de utilización se han publicado una ingente cantidad de artículos de todo tipo sobre el procedimiento y los resultados de esta técnica que han avalado su utilización en el estudio de diversas lesiones mediastínicas y pulmonares.
En el presente artículo se realiza una exhaustiva y ordenada revisión de toda la evidencia científica disponible sobre sus indicaciones más generalizadas, mediante la descripción de las características y los resultados de los estudios específicos sobre su eficacia, seguridad y coste-efectividad disponibles hasta el momento.
Este análisis demuestra que la EBUS-TBNA es una técnica segura, coste-efectiva y con una elevada validez diagnóstica para el diagnóstico y la estadificación mediastínica de pacientes con sospecha o confirmación de cáncer de pulmón, con un grado de evidencia alto. Sin embargo, se precisa de un mayor número de estudios que nos ayuden en la toma de decisiones ante un resultado negativo. La evidencia disponible sobre el papel de la EBUS-TBNA en el diagnóstico de sarcoidosis y neoplasias extratorácicas también es elevada, pero mucho menor cuando la técnica se emplea para el estudio de tuberculosis y de linfoma y para la reestadificación del cáncer de pulmón tras quimioterapia neoadyuvante. No obstante, por su alta seguridad y menor invasividad que las técnicas quirúrgicas, el grado de recomendación para emplear la EBUS-TBNA como la prueba diagnóstica inicial en estas patologías parece muy alto en la mayoría de los casos.
Since it was launched 10 years ago, the widespread implementation of real-time endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in the diagnosis of diseases involving the hilar and/or mediastinal lymph nodes and/or peritracheobronchial lesions has probably been the greatest milestone in the history of bronchoscopy since the introduction of fiberoptic bronchoscopy.1 It was initially used for the diagnosis and staging of lung cancer,2–4 but soon proved useful in other neoplastic and granulomatous processes affecting the mediastinum.1,2,5 In addition to obviating invasive testing procedures, it has had a positive influence on time to diagnosis and improved the cost-effectiveness of lung cancer diagnosis and staging.5–8 Evidence has also been generated on its possible role in the drainage of mediastinal or pleuropericardiac cystic lesions9,10 and in the evaluation of pulmonary vascular disease.11
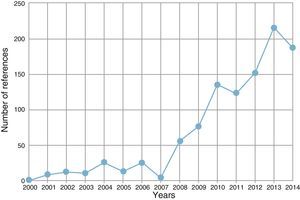
The number of publications supporting the effectiveness and safety of this technique has risen exponentially in recent years (Fig. 1). Numerous meta-analyses and systematic reviews, randomized, cohort and case control studies, case series, and a considerable number of narrative reviews are now available, addressing the effectiveness, efficacy and safety of this technique. In the last five years, many groups have used two different devices to combine the effectiveness of the EBUS-TBNA technique with that of transesophageal ultrasonography-guided fine needle aspiration (EUS-FNA), while others have used one single device, linear endobronchial ultrasound (EUS-B), to investigate lesions via airway and esophageal access routes.12–15
It seems, then, a good moment to consolidate and summarize the scientific evidence on the effectiveness and efficiency of linear EBUS generated during the first 10 years of clinical practice, particularly in the most widely accepted indications.5
We performed a comprehensive, exhaustive review of all publications appearing in the MEDLINE and EMBASE databases on linear EBUS since April 2015, presenting our findings through tables. These tables summarize the characteristics and results of the meta-analyses, or if meta-analyses were not available, of all controlled and other types of studies which examined a specific pathology. We have included the variables that allow the reader to make a clear, overall evaluation of the scientific evidence available on this diagnostic procedure, including sensitivity, specificity, and positive and negative likelihood ratios (PLR and NLR, respectively). In many cases we have calculated likelihood ratios ourselves because, being independent of prevalence, they are more useful than predictive values when comparing different studies.16,17 The I2 index has been included in the meta-analyses: this reflects the total percentage of variation in results caused by the heterogeneity of the study and not by change – the closer the value to 100%, the greater the heterogeneity of the studies in question.18 Finally, the current evidence on safety and cost-effectiveness of the technique is summarized.
In this review, we have deliberately not included studies designed to analyze technical aspects of the EBUS-TBNA procedure, nor have we addressed the processing or yield of the samples for molecular studies: some excellent narrative reviews and recent consensus documents are already available on this topic.19,20
EBUS-TBNA in the Diagnosis and Staging of Lung CancerStaging is an essential aspect of the evaluation of all lung cancer patients, and it acquires a crucial role in the identification of malignant mediastinal invasion by non-small cell lung cancer (NSCLC). EBUS-TBNA is considered a key tool in clinical practice guidelines on mediastinal diagnosis and staging,21–23 and this conclusion is supported by a large quantity of studies. Most of these studies were included in the six meta-analyses published to date,3,4,12,13,21,24 three of which take into account only studies in which EBUS-TBNA was combined with EUS.12,13,21 The characteristics and results of these analyses are summarized in Table 1. A high diagnostic yield for mediastinal endosonography in lung cancer patients is observed. This technique is unquestionably valuable for decision-making, particularly when results are positive, but it is also highly useful when results are negative.
Characteristics and Results of Meta-analyses on the Value of EBUS-TBNA in the Diagnosis and Staging of Lung Cancer.
| Author, Year | Studies (n) | Patients (n) | S (%) | SP (%) | PLR | NLR | I2 |
|---|---|---|---|---|---|---|---|
| Adams, 20093 | 10 | 995 | 88 | 100 | ∞ | 0.12 | NA |
| Gu, 20094 | 11 | 1299 | 93 | 100 | ∞ | 0.07 | 74.4 |
| Dong, 201324 | 9 | 1066 | 90 | 99 | 0.91 | 0.10 | 87.2 |
| Silvestri, 201321 | 26 | 2756 | 89 | 100 | ∞ | 0.11 | NA |
| Silvestri, 201321a | 7 | 811 | 91 | 100 | ∞ | 0.09 | NA |
| Zhang, 201312a | 8 | 822 | 86 | 100 | ∞ | 0.15 | 75.7 |
| Dhooria, 201513a | 10 | 1080 | 91 | 100 | ∞ | 0.09 | 82.4 |
I2: I2 index; NA: not available; NLR: negative likelihood ratio; PLR: positive likelihood ratio; S: sensitivity; SP: specificity.
We found significant variations between the four studies examining this issue, due to differences in the reference tests used, selection methods, study designs, disease prevalence, variations in technique and procedure, experience or possible evaluation in situ by pathologists in the participating centers.2
The meta-analyses which perform a combined analysis of the use of EBUS-TBNA and EUS-FNA all report greater sensitivity for the combined strategy compared to EBUS-TBNA alone. However, a direct comparison of the sensitivity obtained in the individual studies (Table 1) seems to contradict this finding, raising questions as to whether this combination of techniques is routinely required. A very recent specific study tends to support combined use, starting with EBUS-TBNA as the first procedure.25
Three studies26–28 of the direct blinded comparison between EBUS-TBNA and mediastinoscopy, performed in all patients and in different settings, have shown that EBUS-TBNA has a greater or at least similar diagnostic yield (Table 1S, Online Supplement).
Nowadays, attention is mainly focused not only on a comparison between the different techniques, but rather on the most appropriate sequence for applying them and the need for confirming negative results obtained by endosonographic techniques. The ASTER study14 was the first multicenter, randomized trial to compare surgical mediastinal staging with complete endosonography, followed by surgery if negative, in NSCLC patients with mediastinal lymphadenopathies greater than 10mm in diameter on CT or a positive PET (abnormal image of the mediastinum), or in the case of central tumors or suspected N1 involvement (mediastinum normal on imaging, but high suspicion of occult N2 disease). Sensitivity for the diagnosis of lymph node metastasis was 79% for surgical staging and 94% for endosonography followed by surgery in case of negativity. A subsequent Bayesian analysis of the ASTER study29 showed that if the mediastinal imaging results are normal, the post-procedure probability of detecting malignant nodes does not increase if endosonography is followed by surgery, compared to endosonography alone (9% in both cases). When mediastinal involvement is seen on imaging techniques, however, the rate of detection does increase (5% vs 20%), suggesting that only negative results need to be confirmed by surgery. However, these results were not reproduced in the ASTER 2 study, which included NSCLC patients with clinically suspected hilar node involvement,30 so the need for surgical confirmation of negative results when the pre-test probability is low or moderate is still a subject for debate. Various studies have shown that even when changes are observed on CT or PET, the reliability of negative EBUS-TBNA results varies widely, depending on a wide range of variables related with the tumour (type, site, stage, size), the lymphadenopathies (site, echographic features, size, PET avidity), the procedure (number of passes, number and location of the stations sampled, type of sedation), the experience of the endoscopists and pathologists, and the quality of the sample obtained.31,32
More recent clinical practice guidelines, such as those published by the American College of Chest Physicians (ACCP),21 the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR),22 and the European Society of Thoracic Surgeons (ESTS),23 agree on the inclusion of EBUS-TBNA (with or without EUS) as the principal technique for confirming NSCLC mediastinal involvement. However, some of their recommendations on the confirmation of negative results or indications for EBUS-TBNA procedures in patients with normal mediastinum on imaging studies are poorly defined or even divergent (Table 2S Online Supplement).
The value of EBUS-TBNA has been highlighted in a recent randomized multicenter study which reported that the technique had a favorable influence on time to diagnosis and staging and the survival of patients with NSCLC.7
EBUS-TBNA in the Restaging of Non-small Cell Lung Cancer After Induction TreatmentTo date, 5 studies have been published in this specific indication, in a total of 363 patients with histological stages IIIA (N2) (Table 2).33–37 Three of the studies are retrospective, with limited sample size and widely varying rates of mediastinal lymph node involvement (between 20% and 88%) and negative predictive values of EBUS-TBNA (between 22% and 94%) – which was higher when incidence of N2 disease after treatment was low. In general, compared with the benefits of EBUS-TBNA in initial NSCLC staging, the procedure in these cases showed an overall lower sensitivity, and similar specificity and PLR, except in one of the studies, and an NLR that points to a high post-test probability after a negative result. Only Szlubowski et al.37 analyzed the joint efficacy of EBUS and EUS.
Characteristics and Results of Studies Analyzing the Diagnostic Yield of EBUS-TBNA in the Re-Staging of Lung Cancer After Induction Chemotherapy.
| Author, Year | Design | Patients (n) | S (%) | SP (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| Herth, 200833 | Retrospective | 124 | 76 | 100 | ∞ | 0.24 |
| Szlubowski, 201034 | Prospective | 61 | 67 | 86 | 4.79 | 0.38 |
| Zielinsky, 201335 | Retrospective | 88 | 64 | 100 | ∞ | 0.36 |
| Nasir, 201436 | Retrospective | 27 | 50 | 100 | ∞ | 0.50 |
| Szlubowski, 201437 | RCT | 106 | 67 | 96 | 16.75 | 0.34 |
NLR: negative likelihood ratio; PLR: positive likelihood ratio; RCT: randomized clinical trial; S: sensitivity; SP: specificity.
EBUS-TBNA has been frequently used in the diagnosis of patients with central, paratracheal or paraesophageal lesions with no lymphadenopathies and no endobronchial involvement, often after negative results were obtained on other bronchoscopic tests.38 Eight specific studies have been published – almost all retrospective series – in a total of 393 patients who underwent EBUS-TBNA for this indication (Table 3).39–46 In general, the technique appears to have good sensitivity, generally higher than 90%. Specificity is only described in three studies, reported as 100% in all of them, resulting in an NLR which considerably reduces the pre-test probability.
Characteristics and Results of Studies Analyzing the Diagnostic Yield of EBUS-TBNA in the Study of Patients With Intrapulmonary Masses.
| Author, Year | Design | Patients (n) | S (%) | SP (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| Nakajima, 200839 | Prospective | 35 | 94 | 100 | ∞ | 0.06 |
| Tournoy, 200940 | Retrospective | 60 | 82 | ND | ND | ND |
| Zhao, 201341 | Prospective | 66 | 94 | 100 | ∞ | 0.06 |
| Lourido, 201342 | Retrospective | 24 | 96 | ND | ND | ND |
| Bhatti, 201343 | Retrospective | 32 | 94 | ND | ND | ND |
| Verma, 201344 | Retrospective | 37 | 86 | ND | ND | ND |
| Bugalho, 201345 | Prospective | 123 | 90 | 100 | ∞ | 0.10 |
| Dincer, 201546 | Retrospective | 16 | 94 | NA | ND | ND |
NA: not available; NLR: negative likelihood ratio; PLR: positive likelihood ratio; S: sensitivity; SP: specificity.
Up to 30% of extrapulmonary tumors can produce mediastinal or hilar lymph node involvement. In a recent meta-analysis47 of the value of EBUS-TBNA in this indication, which included 6 studies (533 patients), the pooled sensitivity was 85%, specificity was 99%, PLR was 28.6 and NLR 0.16. The most frequently studied primary tumors were: head and neck, colorectal, breast, renal, esophageal, gastric, prostatic, and melanoma. Two studies performed subsequently to this analysis48,49 reported similar results.
EBUS-TBNA in the Diagnosis of LymphomaThe value of EBUS in the study of mediastinal involvement in lymphoproliferative processes is the most controversial indication among those discussed in this review, due to the wide range of published results.50 Seven specific studies on the diagnosis of lymphomas have been published, including 341 patients (Table 4).51–57 The sample size and prevalence are variable (between 10% and 66%), and results on the validity and accuracy of the diagnosis are mostly poor; sensitivity varies widely between the studies (although it is clearly better in cases of recurrence than in primary lymphomas), and the NLR would require negatives to be confirmed. In some of the studies, flow cytometry techniques and immediate evaluation by a pathologist or multidisciplinary committees may have helped to improve the validity of the technique.53,55,56
Characteristics and Results of Studies Analyzing the Diagnostic Yield of EBUS-TBNA in the Study of Lymphoma Patients.
| Author, Year | Design | Patients (n) | S (%) | SP (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| Kennedy, 200851 | Retrospective | 25 | 73 | 100 | ∞ | 0.27 |
| Steinfort, 201052 | Prospective | 55 | 57 | 100 | ∞ | 0.43 |
| Marshall, 201153 | Retrospective | 33 | 100 | NA | NA | NA |
| Iqbal, 201254 | Retrospective | NA | 22 in P55 in R | NA | NA | NA |
| Ko, 201355 | Prospective | 95 | 60 | NA | NA | NA |
| Moonim, 201356 | Prospective | 100 | 88 in P100 in R | 97 | 0.33 | 0.10 |
| Talebian-Yazdi, 201457 | Retrospective | 33 | 55 in P88 in R | NA | NA | NA |
NA: not available; NLR: negative likelihood ratio; P: primary lymphoma; PLR: positive likelihood ratio R: recurrent lymphoma; S: sensitivity; SP: specificity (includes diagnosis and typing).
After lung cancer, most evidence on the role of EBUS-TBNA comes from the study of sarcoidosis. Two meta-analyses have been published recently. The first, by Agarwal et al.,58 includes a selected population with a high previous suspicion of sarcoidosis (prevalence≈80%), while the second, by Trisolini et al.,59 minimizes selection bias by analyzing studies which included patients with suspicious imaging studies showing pathological lymphadenopathies, irrespective of the underlying etiological suspicion (prevalence≈15%). Agarwal et al. looked at 533 patients in 15 studies, while Trisolini et al. studied 2097 patients from 14 publications. Three studies were included in both meta-analyses. The diagnostic yield of both analyses was 79%. In the second study, diagnostic sensitivity was 85% and NLR was 0.16.
A significant number of publications have compared EBUS-TBNA (with/without EUS-FNA) with other standard techniques for investigating sarcoidosis, such as conventional TBNA (cTBNA) and transbronchial biopsy (TBB).60Table 5 summarizes the data from 10 mainly prospective or controlled studies published to date, which include a total of 835 patients.15,61–69 Although most report that EBUS-TBNA provides a better diagnostic yield than cTBNA or TBB, the differences are lower for cTBNA or TBB in stage I disease, particularly for lymphadenopathies greater than 15mm in size and located in right paratracheal or subcarinal stations, and for TBB in stage II disease.15,61–69 Protocolized combination of EUS-FNA with conventional techniques significantly increases their diagnostic yield.15,61–68
Characteristics and Results of Studies Comparing the Diagnostic Yield of EBUS-TBNA With Other Conventional Bronchoscopic Techniques in Sarcoidosis.
| Author, Year | Design | Patients (n) | Sensitivity | ||
|---|---|---|---|---|---|
| cTBNA | TBB | EBUS-TBNA | |||
| Oki, 200761 | Prospective | 15 | 93% | – | 93% |
| Nakajima, 200962 | Retrospective | 35 | – | 31% | 63% |
| Tremblay, 200963 | RCT | 50 | 54% | – | 83% |
| Navani, 201164 | Prospective | 40 | – | 31% | 82% |
| Plit, 201265 | Retrospective | 37 | – | 78% | 84% |
| Von Bartheld, 201315 | RCT | 304 | – | 53% | 66%a |
| Oki, 201266 | Prospective | 62 | – | 37% | 94% |
| Gupta, 201467 | RCT | 130 | 48% | 66–73% | 74.5% |
| Li, 201468 | RCT | 62 | 62% | – | 93% |
| Gnass, 201569 | RCT | 100 | 59% | – | 77%a |
cTBNA: conventional transbronchial needle aspiration; RCT: randomized clinical trial; TBB: transbronchial biopsy.
Six specific studies have been published on the role of EBUS-TBNA in the diagnosis of tuberculosis presenting with mediastinal or hilar lymphadenopathies (Table 6).70–75 These studies, which include just over 550 patients, are mostly case series from individual centers, except for one which is a multicenter study.71 Sensitivity and NLR vary greatly (while specificity and PPR are similar), significantly improving these parameters in some of the articles when the cytological study is combined with meticulous conventional microbiological processing or if molecular techniques are used.74,75
Characteristics and Results of Studies Analyzing the Diagnostic Yield of EBUS-TBNA in the Study of Tuberculosis.
| Author, Year | Design | Patients (n) | S (%) | SP (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| Lin, 200970 | Prospective | 75 | 58.3 | 97 | 0.33 | 0.43 |
| Navani, 201171 | Prospective | 156 | 94 | 100 | ∞ | 0.06 |
| Sun, 201372 | Prospective | 59 | 85 | 100 | ∞ | 0.15 |
| Ren, 201373 | Retrospective | 78 | 64.6 | 100 | ∞ | 0.35 |
| Senturk, 201474 | Prospective | 27 | 90.9 | 100 | ∞ | 0.09 |
| Geake, 201575 | Retrospective | 158 | 62 | 100 | ∞ | 0.38 |
NA: not available; NLR: negative likelihood ratio; PLR: positive likelihood ratio; S: sensitivity; SP: specificity.
Nearly all cost-effectiveness studies focus on lung cancer, and generally conform to three different types.
The first type is based on the application of theoretical probabilistic models, using the results of previously published studies looking at cost savings and determining the most economical strategy among the available alternatives (imaging, endoscopic or surgical techniques). Examples include a study included in the 2011 NICE guidelines for the diagnosis and treatment of lung cancer,76 or a Danish study77 which reported generally in favor of a strategy combining PET with EBUS-TBNA.
The second type would be the cost-benefit studies, based on the experience of the authors themselves or on the literature. In these studies, direct economic analyses are made of cost savings derived from the use of EBUS-TBNA in real or hypothetical cohorts of lung cancer patients, compared to other conventional or surgical methods of diagnosis and staging. At least seven studies of this type from five different countries have been published,8,78–83 of which 6 report in favor of EBUS-TBNA, but the costs saved vary widely depending on the study design and the health system of each country (Table 3S Online Supplement). In the only study in which the costs of mediastinoscopy were slightly lower than those of EBUS-TBNA, this procedure was conducted in the operating room with general anesthesia.83
The third group of studies are cost-effectiveness analyses of quality of life and survival, such as the study performed on the ASTER study cohort84 in the United Kingdom, Netherlands and Belgium. Results favored EBUS-TBNA, reporting lower overall costs and better quality of life for patients, compared to surgical techniques.
EBUS-TBNA ComplicationsAs the use of this technique has expanded widely in the last ten years, various complications associated with the procedure have been published in individual reports or in small case series: most complications have been rare or lacking in severity. We have identified three studies from which we were able to extract an overall picture of the safety of this technique.
Von Bartheld et al.85 made a systematic review of 9119 patients, and reported a severe complication rate of 0.14%, the most prevalent being infections and pneumothorax (both 0.02%), predominantly in patients with cystic lesions and sarcoidosis. No deaths were reported.
Asano et al.,86 in a national survey performed in Japan on EBUS procedures in 7345 patients, reported a prevalence of severe complications of 1.23%, the most common being bleeding (0.68%), followed by infectious complications (mediastinitis, abscesses, etc.) (0.17%), respiratory failure (0.07%), and finally pneumothorax (0.03%). One death was reported.
Finally, in the AQUIRE87 study, a prospective registry including 1317 patients from six hospitals in the United States, the incidence of severe complications was 1.44%, mostly pneumothorax (0.53%), followed by respiratory failure (0.23%) and bleeding (0.2%). One death was reported. Conventional techniques, such as TBB, were also included in this study: TBB was identified as an independent risk factor for the development of complications.
ConclusionsFrom the findings of our review, we concluded that EBUS-TBNA is a safe, cost-effective technique with a high grade of evidence and a valuable tool in the diagnosis and mediastinal staging of patients with suspected or confirmed lung cancer. However, more studies are needed to guide decision-making in the event of a negative result.
Evidence on the role of EBUS-TBNA in the diagnosis of sarcoidosis and extrathoracic tumors is also high, but the yield of the technique is much lower in the study of tuberculosis and lymphoma and in the restaging of lung cancer after neoadjuvant chemotherapy. Nevertheless, due to its good safety record and lack of invasiveness compared to surgical techniques, the grade of evidence for recommending EBUS-TBNA as the initial diagnostic test in patients with these diseases is very high in most cases.
Conflict of InterestsThe authors declare that they have no conflict of interests with regard to the content of this manuscript.
Please cite this article as: Fernández-Villar A, Mouronte-Roibás C, Botana-Rial M, Ruano-Raviña A. Diez años de ecobroncoscopia lineal: evidencia sobre su eficacia, seguridad y coste-efectividad. Arch Bronconeumol. 2016;52:96–102.