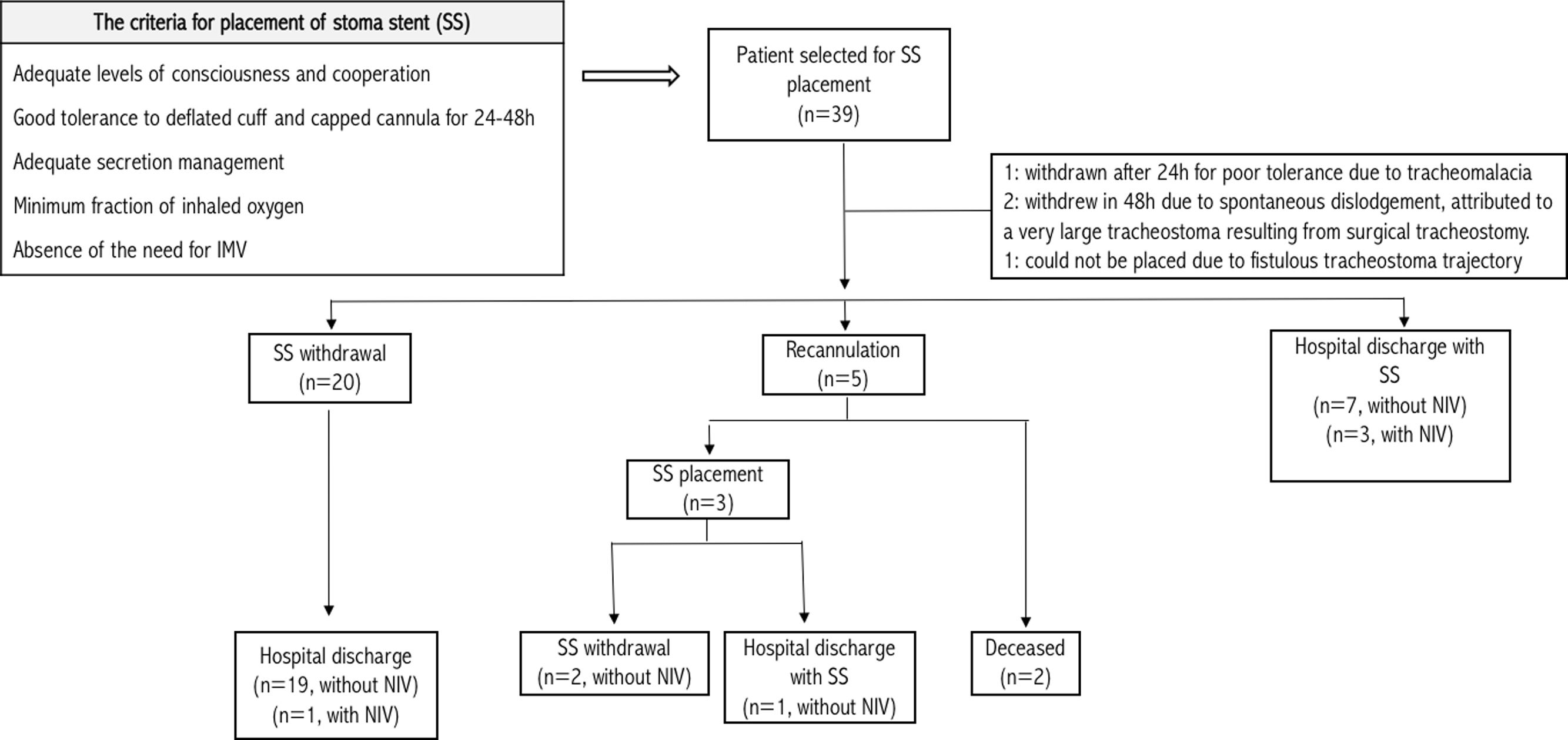
A systematic review on decannulation protocols used in international studies was published in 2019.1 While some decannulation practices are well described, there is no consensus regarding the decannulation algorithm. Recent publications have highlighted the stoma stent (SS) placement in the decannulation process.2,3 However, existing literature, often retrospective or with limited patient samples, shows variability in studied populations, SS types, and decannulation protocols, potentially limiting SS adoption in decannulation procedures. We hypothesized that use of the Montgomery cannula (MC) in high-risk patients would reduce the risks of reintubation. The objective of this study was to evaluate recannulation rates in tracheostomized patients, in whom a MC was placed. We report the first cases of MC placement in our center and the largest published cohort analyzing the use of MC as a tool during the decannulation procedure in high-risk patients.
In this retrospective study, we evaluated 39 patients admitted to a conventional hospitalization unit but in our area of monitoring and specialized care (Pneumology Department), over last 5-years. The decision to use a SS in the decannulation process was based on a clinical judgment considering a safe method for decannulating tracheostomized patients with prolonged length of hospital stay and critical illness myopathy. These patients had suffered multiple medical or surgical complications or risk factors for mechanical ventilation reestablishment, such as obesity, respiratory history, or previous hypercapnia. The criteria for determining the appropriate timing for SS placement were based on our unit's decannulation protocol, which conforms to practices commonly described in the literature.4–7 The device used was a short-term MC (Boston Medical Products, Massachusetts), number 8 or 6 depending on the patient, placed with fibreoptic endoscopic evaluation. Detailed medical record review of the identified cases was conducted. Successful decannulation was defined as replacement of the tracheal cannula (TC) with a SS without the need for recannulation. Ethical approval (IIBSP-MON-2023-126) was obtained from Hospital de la Santa Creu i Sant Pau. Baseline characteristics were compared using unpaired t-test or Mann–Whitney U test for continuous variables and Chi-squared test for binary variables. Analysis of variance was used to identify predictors of unfavorable clinical outcomes, with significance set at p<0.05. Statistical analyses were conducted using SPSS version 26 and R software.
Among 39 patients with prolonged weaning, finally the MC was correctly placed in 35 patients (Fig. 1). Mean (SD) age was 65 (10.5) years. Following data are expressed as median, first quartile (Q1) and third quartile (Q3), the duration of intubation was 21 (12–38) days. Nine of them had been intubated due to ARDS, others due to infectious cause (n=8), hypercapnic respiratory failure (n=5), multiorgan failure (n=2), neurological cause (n=2) and surgical intervention (n=1). The remaining patients had been intubated due to multiple causes. Up to 69.2% of patients had respiratory comorbidities. Chronic airflow limitation (17.9%), neuromuscular disease (17.9%) and post-COVID sequelae (15.4%) were the most frequent respiratory comorbidities. The TC was removed after 34 (21–63) days; the MC was inserted during 15 (5–36) days and the median length of hospital stay was 87 (61–141) days.
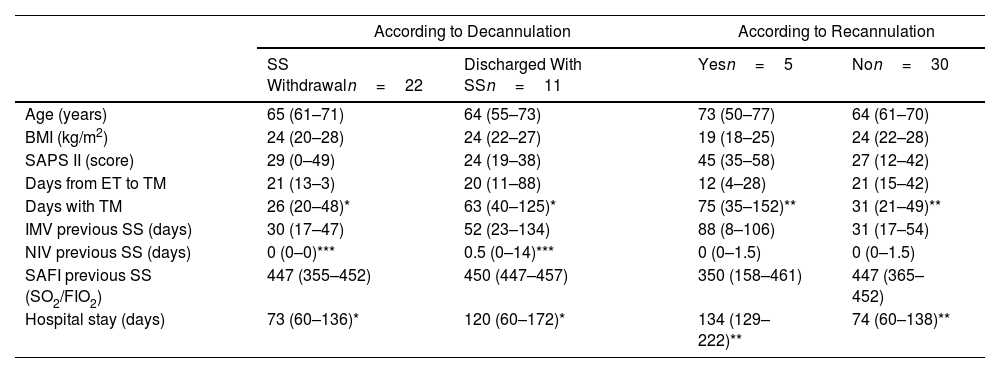
Decannulation was successful in most of cases (94%) and reintubation was avoided in 14% (Fig. 1). Recannulation with a TC was required due to emergency surgery (n=1), benzodiazepine intoxication (n=1), and respiratory distress from abundant mucopurulent secretions (n=3). One of these patients died from a postsurgical complication and another one from an unfavorable prognosis of a neuromuscular disease. No complications related to the MC were recorded apart from the cases where placement was unsuccessful. Prior to hospital discharge, cases discharged with SS (n=11) were discussed among the interdisciplinary team. Sixty percent of them had neuromuscular diseases complicated by critical illness myopathy, occasionally hampering optimal secretion management. Others presented respiratory infections from multidrug-resistant microorganisms. Comparing discharged patients with and without SS, the main predictive factors were the duration of TC use (p=0.014) and non-invasive ventilation (NIV) (p=0.01) prior to SS placement. Recannulated patients had significantly longer durations of TC use (p<0.01) and hospital stay (p<0.01) (Table 1). The device was removed in 100% of cases within two months following hospital discharge, with no deaths or complications due to MC reported. One-year survival rate of patients discharged was 60%.
Patients Clinical Profile With Stoma Stent According Different Outcomes.
| According to Decannulation | According to Recannulation | |||
|---|---|---|---|---|
| SS Withdrawaln=22 | Discharged With SSn=11 | Yesn=5 | Non=30 | |
| Age (years) | 65 (61–71) | 64 (55–73) | 73 (50–77) | 64 (61–70) |
| BMI (kg/m2) | 24 (20–28) | 24 (22–27) | 19 (18–25) | 24 (22–28) |
| SAPS II (score) | 29 (0–49) | 24 (19–38) | 45 (35–58) | 27 (12–42) |
| Days from ET to TM | 21 (13–3) | 20 (11–88) | 12 (4–28) | 21 (15–42) |
| Days with TM | 26 (20–48)* | 63 (40–125)* | 75 (35–152)** | 31 (21–49)** |
| IMV previous SS (days) | 30 (17–47) | 52 (23–134) | 88 (8–106) | 31 (17–54) |
| NIV previous SS (days) | 0 (0–0)*** | 0.5 (0–14)*** | 0 (0–1.5) | 0 (0–1.5) |
| SAFI previous SS (SO2/FIO2) | 447 (355–452) | 450 (447–457) | 350 (158–461) | 447 (365–452) |
| Hospital stay (days) | 73 (60–136)* | 120 (60–172)* | 134 (129–222)** | 74 (60–138)** |
Data are shown as median values and quartiles.
BMI: body mass index; ET: endotracheal intubation; TM: tracheotomy; IMV: invasive mechanical ventilation; NIV: non-invasive ventilation; SAPS: simplified acute physiology score; SAFI: arterial oxygen saturation/fraction of inhaled oxygen; SS: stoma stent.
While capping and placing smaller calibre tracheostomy cannulas appears to be the most commonly used approach to decannulation. This procedure causes increased airway resistance and may lead to increased breathing effort. With the SS, airway resistance is of no clinical relevance.8 It is worth highlighting that recannulation after a SS placement is straightforward and safe. Thus rendering unnecessary the need for general anesthesia and reintubation, reduces associated risks. Additionally, avoiding patient transfer to an intensive care unit (ICU) ensures continuity of care required for recovery and it allows for family accompaniment. We believe the SS was a key factor for our patients’ favorable outcomes. Despite the limitations of our study, it is important to highlight that 1-year survival rate exceeds that reported in other publications with similar population.9 To date, the MC has been evaluated in a retrospective study, as a tool leading to tracheostome closure in a limited number of cases.10 A more recent publication with a small sample of 14 patients reported a successful decannulation rate (92%) similar to that of our cohort.3 In a study of tracheostomy retainer (TR) (Teleflex Medical Inc., Germany) use in a cohort of 384 patients,2 the percentage of patients in whom the TR could not be placed was similar to our reported percentage of MC that could not be placed. And, as in our study, that study described secretions as one of the main reasons for recannulation. However, the recannulation rate (28%) in that study was higher than in our study. Note that unlike the MC, the TR requires neck tapes to secure the device and use is not recommended beyond a week. Furthermore, the metallic flap that occludes the proximal end of the TR prevents it from being opened when needed and means that its use is incompatible with magnetic resonance imaging (MRI) scans.
Determining clinical predictors of decannulation success is crucial. Some authors have proposed clinical signs, such as swallowing and cough, as predictors.1,5 While Budweiser et al.2 referred to the duration of spontaneous breathing prior to decannulation as an indicator of the need for recannulation, this is a variable that tends to differ greatly between studies. In our cohort, the duration of TC use and the length of hospital stay can be considered predictors of recannulation. Also, considering the characteristics of recannulated patients, factors such as patient age, BMI, severity upon admission to the ICU, and oxygenation status prior to SS placement should be taken into account as selection criteria for SS candidates.
The main limitation of this study was the retrospective design in a single centre, and the lack of randomized control to confirm noninferiority of the MC in decannulation. Nevertheless, the results provide valuable insights into the use of SS during the decannulation process, and indicate potential directions for future research. Additionally several studies have shown that the MC is a safe device in the patient's home.10–13
Stoma stent placement is a viable and safe procedure that reduces the risk of orotracheal reintubation when decannulating patients who have undergone prolonged length of hospital stay and critical illness myopathy. The difference between our results and those obtained in other similar studies highlights the lack of uniformity in the management of this type of patient. Since our results are merely observational, they need to be validated in a prospective clinical trial.
Conflict of InterestsThe authors state that they have no conflict of interests.