Treatment of chronic hypercapnic failure in COPD patients with home noninvasive ventilation (HNIV) remains unclear.
AimTo create a curated cohort of all COPD patients on HNIV in Catalonia, perform a cluster analysis, and evaluate mortality evolution.
Study design and methodsThis study was a multicenter, observational study including all COPD patients on HNIV on 1st January of 2018. Patients were selected through the Catalan Health Service, and administrative and clinical data were obtained in the previous four years. Principal component analysis of mixed data and hierarchical clustering were performed to identify clusters of patients. Mortality was evaluated from 1 January 2018 until 31 December 2020.
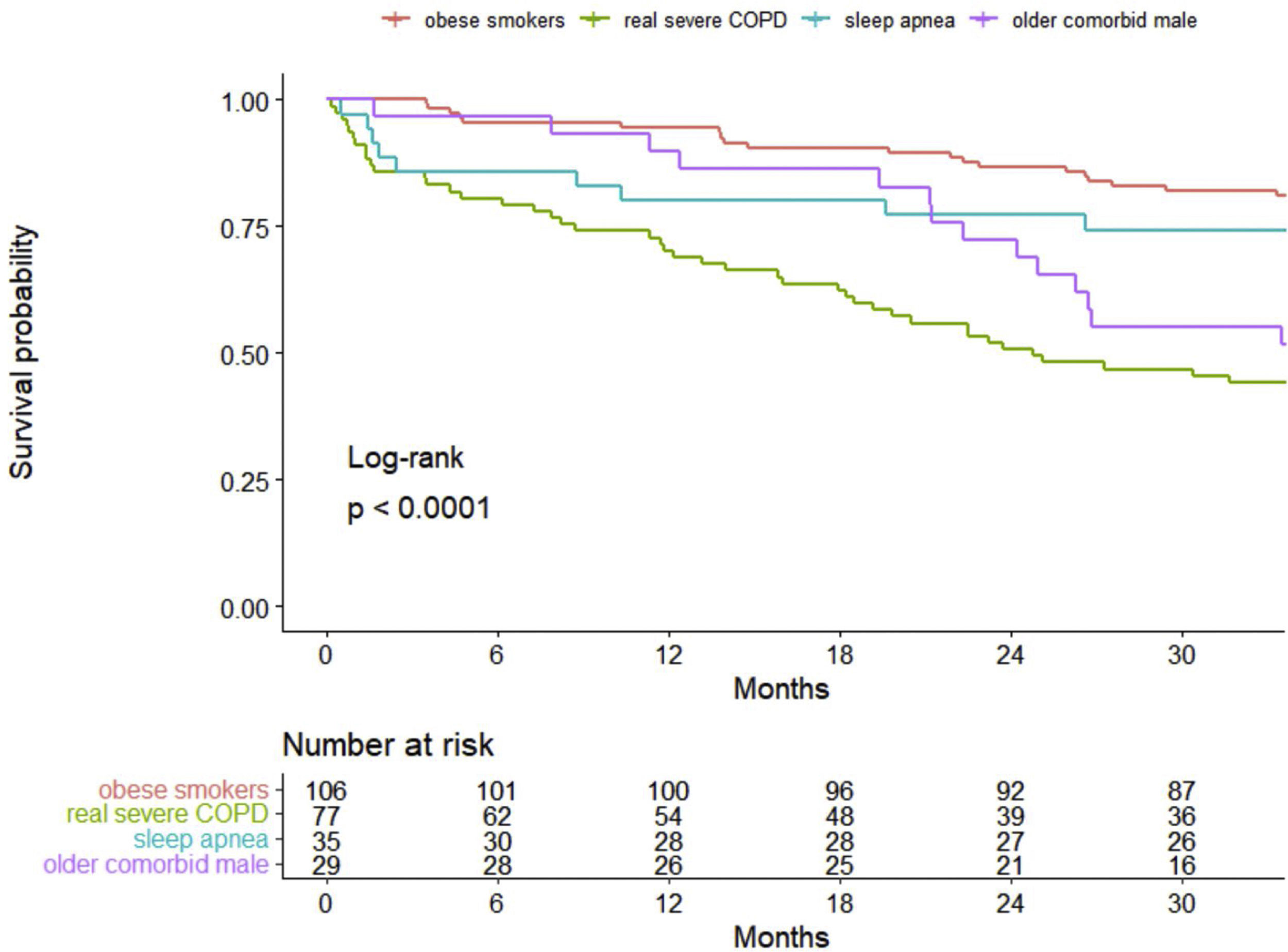
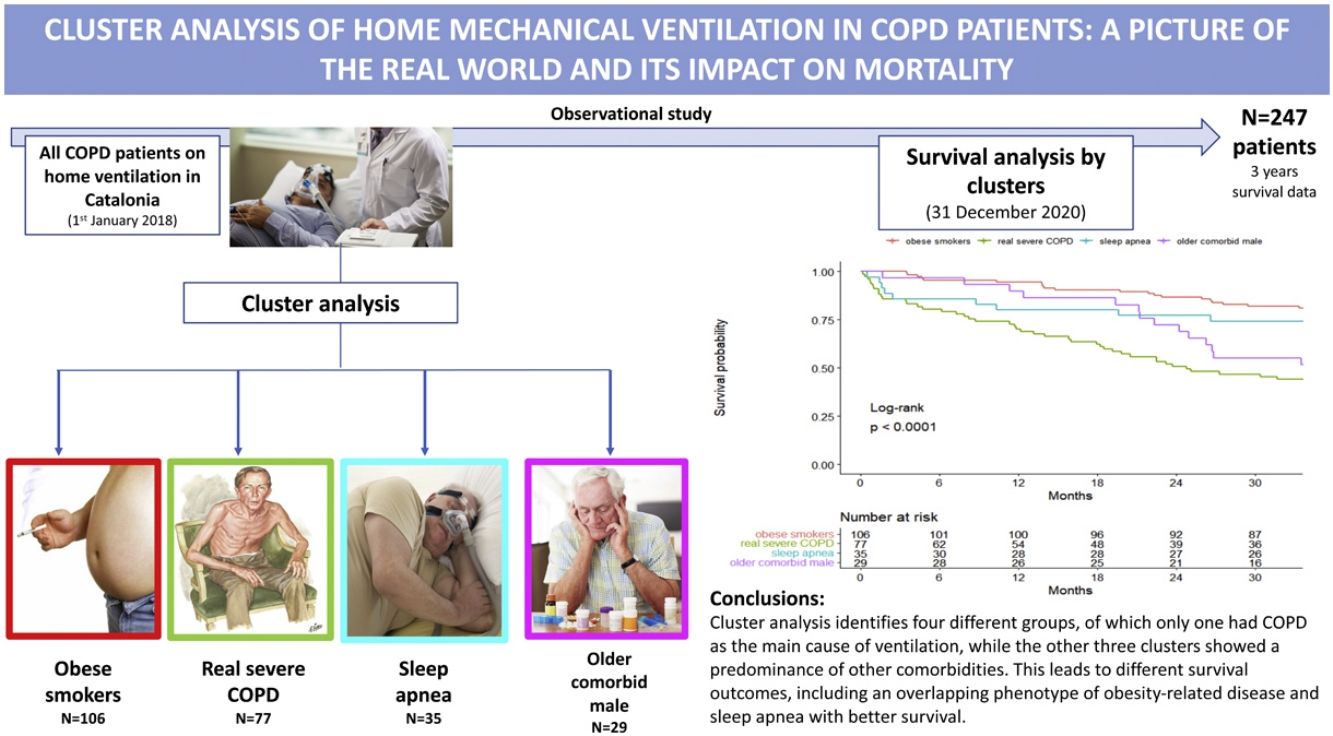
ResultsA total of 247 patients were enrolled. They were mostly male (78.1%), with a median (SD) age of 70.4 (9.4) years old. In 60%, 55% and 29% of patients, obesity, sleep apnea and heart failure coexisted, respectively. Cluster analysis identified four well-differentiated groups labeled for their clinical characteristics: (1) obese smokers, (2) very severe COPD, (3) sleep apnea and (4) older comorbid males. Patients belonging to Clusters (2) and (4) had a worse prognosis than patients in Clusters (1) and (3).
InterpretationA high heterogeneity in the prescription of HNIV was demonstrated. Cluster analysis identifies four different groups, of which only one had COPD as the main cause of ventilation, while the other three clusters showed a predominance of other comorbidities. This leads to different survival outcomes, including an overlapping phenotype of obesity-related disease and sleep apnea with better survival.
Chronic obstructive pulmonary disease (COPD) continues to be one of the leading causes of death and disability worldwide,1 ranking fourth in the world's leading causes of death. Several therapeutic options have emerged, but to date, only smoking cessation and long-term oxygen therapy (LTOT) have been shown to increase survival.2 However, patients with more severe COPD continue to be a challenge due to their greater disability and consumption of medical resources, noting that once hypercapnia develops, 2-year mortality increases by approximately 30–40%.3
In COPD patients with chronic hypercapnic failure, nocturnal noninvasive positive pressure ventilation (NIPPV) has been postulated as a potentially beneficial therapy. There are several theories that explain why NIPPV could be beneficial in these types of patients, including improvement in sleep and time efficiency,4 amelioration of nocturnal hypoventilation,5 recovery of inspiratory muscle function6 and a decrease in hyperinflation leading to an improvement in respiratory mechanics.5,7 However, the effects of chronic NIPPV in stable hypercapnic COPD patients in randomized clinical trials, retrospective studies and systematic reviews are contradictory in terms of benefits on survival rates, reductions in hospital admissions, decreased carbon dioxide retention and improvements in symptoms or quality of life.8–15 This may be due to long recruitment periods, heterogeneity of the centers involved and their therapeutic strategies, variability in the exclusion criteria, small numbers of patients and uncontrolled designs.
Despite the lack of clear evidence from randomize control trials (RCTs), the number of COPD patients on home noninvasive ventilation (HNIV) has increased progressively worldwide. RCTs have high internal validity but often represent fewer than 5% of patients treated in routine care.16 Therefore, it is difficult to extrapolate the results to the general population, and furthermore, there are retrospective studies based on daily clinical practice showing that long-term NIPPV reduces hospital readmissions after acute exacerbation in addition to other benefits,13 so other study designs could be very enlightening. Additionally, in the real world, it is sometimes difficult to define whether NIPPV has been prescribed due to worsening of COPD or has been prescribed for other causes in patients who have COPD.
We created a curated cohort of all COPD patients ventilated in Catalonia to describe patient characteristics from multiple perspectives and to perform a cluster analysis of this cohort to evaluate its different clinical and mortality evolution.
Materials and methodsStudy design and populationThis is a multicenter, retrospective and prospective observational study including all COPD patients on HNIV in Catalonia on 1 January 2018, involving a total of 12 hospitals.
HNIV in Catalonia is funded through the Catalan Health Service (CatSalut). The estimated prevalence of publicly funded HNIV in 2018 was 47.5 patients per 100,000 population (Source: AQuAS), a total of 3550 patients. For our study, COPD patients on HNIV were first identified using the CatSalut billing database on 1 January 2018. The clinicians of the participating hospitals compared this administrative sample with their hospital data and confirmed the COPD diagnosis criteria (smoking habit, symptoms and lung function tests) and HNIV treatment. The presence and severity of COPD was determined using criteria of the Global Initiative for Chronic Obstructive Lung Disease1 (GOLD; post bronchodilation forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio<70%). We included patients with poor life expectancy related to COPD and patients with a high number of comorbidities. No exclusion criteria were used if COPD diagnosis was confirmed by a hospital.
A total of 247 patients with COPD and HNIV from 12 hospitals in Catalonia were enrolled. The study was approved by the Medical Ethics Committee of Hospital Clinic (CEIC/HCP/2017/0103). Written informed consent was acquired from each participant following a detailed explanation of the study purposes.
Data collectionClinical dataAt baseline, the main source of raw clinical/administrative data was from the Agency for Health Quality and Assessment of Catalonia (AQuAS). These administrative data contained information about the main diagnosis and HNIV start date. Data about prescription drug and health care resource consumption (exacerbations and hospitalizations, visits to general practitioners, referrals to specialists and emergency room visits) were obtained over the previous four years (period from 2014 to 2017).
Collected data included demographic information, social economic index for ABS (basic health area in Catalonia) in 2017, GOLD 2017 classification, lung function test and arterial blood gases (ABG) on room air (available in medical records in the last year prior to the HNIV), HNIV efficacy (ABG under HNIV and oximetry values), symptoms questionnaires (CAT and mMRC), body mass index and comorbidities were recorded. Long-term oxygen therapy (LTOT) and ventilator use (time since the start of ventilation, airway access and interfaces, device model and ventilator mode and settings, such as inspiratory/expiratory pressure, respiratory rate, tidal volume) and prescribed time of use were assessed. The diagnosis of sleep apnea was made on the basis of standard medical care (more than 15 events per hour in a sleep study). Encoding personal and clinical identifiers (CIPs) ensured the confidentiality of the information.
Survival outcomeSurvival was calculated prospectively from 1st January 2018 to the end of the study (31 December 2020) or death. Death has been considered regardless of the cause.
Statistical methodsCharacteristics for all patients are presented as the mean (standard deviation) or number (percentage).
A preliminary step before clustering was to impute missing values in the variables selected for the cluster analysis. Variables selected for the cluster analysis are provided in e-Table 1. Briefly, variables included were age, sex, smoking, type of mask, inspiratory pressure, LTOT, years with HNIV, the GOLD 2017 classification, social economic index, comorbidities and clinical data, such as prescription drug use and health care resource consumption. Because we were had quantitative and qualitative data, the principal component method of factorial analysis for mixed data (FAMD) was used for imputation.17
We combined principal component analysis for mixed data18 and hierarchical clustering as a technique to identify groups of similar patients. In the first step, PCA for mixed data (principal component method that allows quantitative and qualitative data) was used to reduce the number of variables while retaining as much variability as possible from the original data. We used a screen plot to explore the number of principal components that should be kept for the analyses (eigenvalue>1). Furthermore, the contribution attributed to variables reflects their influence on the composition of each principal component. In the second step, the selected principal components (in the previous step) were uncorrelated and used in hierarchical clustering, carried out for determining clusters of patients. Ward's method minimizes the total variance within clusters. Visual assessment of the dendrogram was used to identify the optimal number of clusters.
Overall survival was analyzed using Kaplan–Meier curves, and the log-rank test was used to compare the survival curves between the clusters.
All tests were two-sided, and a P value of 0.05 was considered statistically significant. R statistical software, version 4.0.1 (R Project for Statistical Computing), was used for all analyses.
ResultsPatient characteristicsA total of 247 COPD patients on HNIV were included. The characteristics of the study population are displayed in Table 1. They were mostly male (78.1%), with a median (SD) age of 70.4 (9.38) years old. Most were former smokers; however, 27.1% of patients actively smoked at the time of the study. Regarding lung function, the median (SD) FEV1 and FVC was 36.6% (13.4) and 54% (14.1), respectively, with 32% of patients classified as having severe COPD (<30% of FEV1). Additionally, more than half of the patients were classified as GOLD D. The mean (SD) baseline arterial blood gases were 7.37 (0.07), 60.4 (18.2) mmHg, and 60.4 (13) mmHg for pH, PaO2 and PaCO2, respectively.
Characteristics of COPD patients using Home Mechanical Ventilation in Catalonia (N=247).
| Global N=247 | |
|---|---|
| Sociodemographic characteristics | |
| Age (years) | 70.4 (9.38) |
| Sex | |
| Female | 54 (21.9%) |
| Male | 193 (78.1%) |
| Smoking habit | |
| Nonsmokers | 2 (0.85%) |
| Former smokers | 170 (72.0%) |
| Smokers | 64 (27.1%) |
| Socioeconomic indexa | 40.4 (15.6) |
| Ventilation parameters | |
| Type of mask | |
| Nasal | 35 (14.6%) |
| Nasal-mouth | 205 (85.4%) |
| IPAP pressure (cm H2O) | 19.2 (2.80) |
| EPAP pressure (cm H2O) | 7.47 (2.05) |
| Home Non-Invasive ventilation (years) | 3.73 (3.28) |
| Clinical assessment | |
| GOLD classification (2017): | |
| A | 21 (9.21%) |
| B | 44 (19.3%) |
| C | 35 (15.4%) |
| D | 128 (56.1%) |
| CAT | 16.5 (8.24) |
| Dyspnea (mMRC) | 3.56 (1.08) |
| Prescription drugsb(2014–2017) | |
| Alimentary tract and metabolism | 468 (273) |
| Nervous system | 60.1 (60.4) |
| Cardiovascular system | 75.9 (95.3) |
| Respiratory system | 109 (99.8) |
| Primary care visitsb | 76.3 (57.4) |
| Emergency visitsb | 10.8 (9.92) |
| Hospital admissions | |
| Number of hospital admissions (2016 and 2017) | 1.88 (2.22) |
| Admitted to the ICU: Yes | 41 (17.1%) |
| Comorbidities | |
| Obesity | 148 (59.9%) |
| Heart failure | 71 (28.7%) |
| Neuromuscular disease | 7 (2.83%) |
| Sleep apnea | 136 (55.1%) |
| Rib cage alteration | 43 (17.4%) |
| Hypertension | 164 (66.4%) |
| Lung function | |
| FEV1(%) | 36.6 (13.4) |
| <30 | 76 (32.1%) |
| 30–49 | 118 (49.8%) |
| 50–79 | 42 (17.7%) |
| ≥80 | 1 (0.42%) |
| FVC (%) | 54.0 (14.1) |
| Baseline arterial gasometry | |
| pH | 7.37 (0.07) |
| PaO2(mmHg) | 60.4 (18.2) |
| PaCO2(mmHg) | 60.3 (13.0) |
The results are presented as the number of participants (%) and mean (SD). Numbers do not add due to missing values. IPAP: inspiratory positive airway pressure, GOLD: Global initiative for chronic Obstructive Lung Disease, ICU: Intensive Care Unit, FEV1: forced expiratory volume, FVC: forced vital capacity, CAT: COPD assessment test, mMRC: modified Medical Research Council.
This was a comorbid cohort with 66.4% of patients having hypertension, 59.9% having obesity, 55.1% having sleep apnea and 28.7% having heart failure. This is resulted in the regular use of drugs other than drugs for the respiratory system (Table 1). Moreover, primary health center and emergency department visits were high (mean (SD): 76.3 (57.4) and 10.8 (9.92), respectively), as well the number of hospitalizations (mean (SD): 1.88 (2.22)) and ICU admissions (17.1%).
The patients had used HNIV for a mean (SD) of 3.73 (3.28) years, most frequently using nasal mouth masks (85.4%). The mean (SD) IPAP pressure was 19.2 (2.80), and 74.2% of patients were using LTOT.
Cluster analysisCluster analysis was performed on the variables provided and described in e-Table 1 using PCA for mixed data followed by Ward's hierarchical clustering procedure. The first 10 principal components selected (eigenvalues>1) accounted for 64% of the variability (e-Fig. 1). Contributions (in percentage) of the selected variables to each principal component are shown in e-Fig. 2. Classification of the patients using hierarchical clustering based on the 10 principal components resulted in a dendrogram that showed four clusters (e-Fig. 3).
Table 2 presents the characteristics of the patients according to the four clusters defined above. Cluster One (1) was mainly characterized by obese and GOLD D patients (73.6% and 46.2%, respectively), and 67% of these patients were using LTOT. This was the group with the highest percentage of current smokers (41.5%). This cluster was then labeled as “obese smokers”. In contrast, Cluster Two (2) was predominantly GOLD D and males (89.6% and 89.6%, respectively) using LTOT (89.6%). They presented the worst pulmonary function (mean FEV1: 30.7%), with a high proportion of severe patients (FEV1<30%: 50%) and a high number of hospital admissions. This cluster was labeled as “very severe COPD”. Cluster Three (3) was composed of obese and male patients (71.4% and 91.4%, respectively) with sleep apnea (74.3%) and less severe GOLD classification, with almost 60% of the patients being GOLD A/B/C with the lowest percentage of oxygen at home (60%). This cluster was labeled “sleep apnea”. Finally, Cluster Four (4) was characterized by older males (mean (SD) age of 76.8 (8.78) years old), with heart failure (58.6%), severe COPD (44.8% GOLD D D) and predominantly using LTOT (79.3%). This cluster also had a higher number of primary health center visits with a greater consumption of alimentary tract and metabolism, nervous system and cardiovascular drugs. This cluster was labeled “older comorbid male”.
Characteristics of the patients according to the four clusters.
| Obese smokers (1)N=106 | Very severe COPD (2)N=77 | Sleep apnea (3)N=35 | Older comorbid male (4)N=29 | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age (years) | 67.4 (8.81) | 72.6 (8.49) | 68.9 (9.84) | 76.8 (8.78) |
| Sex | ||||
| Female | 39 (36.8%) | 8 (10.4%) | 3 (8.57%) | 4 (13.8%) |
| Male | 67 (63.2%) | 69 (89.6%) | 32 (91.4%) | 25 (86.2%) |
| Tobacco habit | ||||
| Nonsmokers/Former smokers | 62 (58.5%) | 67 (87.0%) | 28 (80.0%) | 26 (89.7%) |
| Smokers | 44 (41.5%) | 10 (13.0%) | 7 (20.0%) | 3 (10.3%) |
| Socioeconomic indexa | 39.3 (15.6) | 40.4 (16.7) | 42.4 (16.5) | 41.7 (11.1) |
| Ventilation parameters | ||||
| Type of mask | ||||
| Nasal | 19 (17.9%) | 4 (5.19%) | 4 (11.4%) | 8 (27.6%) |
| Nasal-mouth | 87 (82.1%) | 73 (94.8%) | 31 (88.6%) | 21 (72.4%) |
| IPAP pressure (cm H2O) | 19.6 (2.13) | 19.4 (3.63) | 18.5 (2.49) | 18.8 (3.82) |
| EPAP pressure (cm H2O) | 7.6 (1.98) | 6.9 (2.18) | 7.6 (2.00) | 7.9 (1.87) |
| Long-term oxygen therapy (Yes) | 71 (67.0%) | 69 (89.6%) | 21 (60.0%) | 23 (79.3%) |
| Home mechanical ventilation (years) | 3.16 (2.70) | 4.05 (4.20) | 3.63 (2.77) | 5.06 (2.64) |
| Clinical assessment | ||||
| GOLD classification (2017): | ||||
| A | 3 (2.83%) | 0 (0.00%) | 17 (48.6%) | 1 (3.45%) |
| B | 26 (24.5%) | 5 (6.49%) | 2 (5.71%) | 12 (41.4%) |
| C | 28 (26.4%) | 3 (3.90%) | 1 (2.86%) | 3 (10.3%) |
| D | 49 (46.2%) | 69 (89.6%) | 15 (42.9%) | 13 (44.8%) |
| CAT | 15.4 (7.85) | 20.1 (6.96) | 13.2 (9.45) | 16.8 (8.73) |
| Dyspnea (mMRC) | 3.29 (1.09) | 4.07 (0.89) | 3.00 (1.10) | 3.85 (0.88) |
| Prescription drugsb(2014–2017) | ||||
| Alimentary tract and metabolism | 39.5 (39.4) | 54.6 (48.0) | 48.7 (43.1) | 159 (73.6) |
| Nervous system | 76.2 (105) | 74.0 (88.9) | 62.6 (77.8) | 94.7 (86.2) |
| Cardiovascular system | 81.2 (72.6) | 89.5 (76.6) | 108 (78.8) | 251 (136) |
| Respiratory system | 102 (60.8) | 160 (112) | 96.3 (72.5) | 143 (76.2) |
| Primary care visitsb | 55.4 (36.9) | 89.9 (64.3) | 68.1 (38.1) | 126 (77.5) |
| Emergency visitsb | 7.55 (7.82) | 16.2 (12.1) | 6.94 (6.43) | 10.3 (7.03) |
| Hospital admissions | ||||
| Number of hospital admissions (2016 and 2017) | 1.24 (1.36) | 3.27 (2.91) | 0.91 (0.98) | 1.62 (1.88) |
| Admitted to the ICU: Yes | 4 (3.77%) | 8 (10.4%) | 24 (68.6%) | 6 (20.7%) |
| Comorbidities | ||||
| Obesity | 78 (73.6%) | 16 (20.8%) | 25 (71.4%) | 29 (100%) |
| BMI (kg/m2) | 34.5 (7.50) | 27.8 (5.27) | 34.2 (6.92) | 36.9 (5.98) |
| Heart failure | 26 (24.5%) | 19 (24.7%) | 9 (25.7%) | 17 (58.6%) |
| Neuromuscular disease | 0 (0.00%) | 7 (9.09%) | 0 (0.00%) | 0 (0.00%) |
| Sleep apnea | 56 (52.8%) | 28 (36.4%) | 26 (74.3%) | 26 (89.7%) |
| Rib cage alteration | 15 (14.2%) | 22 (28.6%) | 3 (8.57%) | 3 (10.3%) |
| Lung function | ||||
| FEV1(%) | 38.6 (12.8) | 30.7 (10.9) | 40.2 (15.8) | 40.6 (14.3) |
| <30 | 24 (23.5%) | 37 (50.0%) | 9 (27.3%) | 6 (21.4%) |
| 30–49 | 56 (54.9%) | 32 (43.2%) | 16 (48.5%) | 14 (50.0%) |
| 50–79 | 22 (21.6%) | 5 (6.76%) | 7 (21.2%) | 8 (28.6%) |
| ≥80 | 0 (0.00%) | 0 (0.00%) | 1 (3.03%) | 0 (0.00%) |
| FVC (%) | 56.4 (13.8) | 49.0 (12.4) | 58.9 (16.6) | 52.1 (12.5) |
The results are presented as the number of participants (%) and mean (SD). Numbers do not add due to missing values. IPAP: inspiratory positive airway pressure, GOLD: Global initiative for chronic Obstructive Lung Disease, ICU: intensive care unit, CAT: COPD assessment test, mMRC: modified Medical Research Council.
To interpret the clusters and compare their features, we created a plot summarizing some of the main differences (one line per cluster) (e-Fig. 4).
Survival analysisThe overall percent of death among all patients at 3 years of follow-up was 34.8%, with 28.3 (12.0) (mean (SD)) months of follow-up. Kaplan–Meier curves of survival are shown in Fig. 1. Clearly, patients belonging to Clusters 2 (very severe COPD) and 4 (older comorbid male) had a worse prognosis than patients in Clusters 1 (obese smokers) and 3 (sleep apnea). The log-rank test indicated significant differences in survival curves among clusters.
DiscussionIn the real world, the number of COPD patients receiving HNIV is growing. However, RCTs show controversial results to support the indication of HNIV to treat chronic respiratory failure in COPD patients. To our knowledge, this is the first well-characterized cohort including 247 ventilated severe COPD patients with large administrative data and long-term follow-up, allowing us to describe what happens in real practice.
The main result of this COPD severe cohort was the high heterogeneity of the HNIV prescription, with more than half of the patients having coexisting obesity (59.9%) and sleep apnea (55.1%). Moreover, 25% of patients were on HNIV only, without long-term oxygen therapy. The latter were frequently obese (33%) and with sleep apnea (40%). Selecting the appropriate variables, cluster analysis determined four representative distinct groups of patients: Cluster One (1) was composed of obese smokers with a high proportion using LTOT; Cluster Two (2) had the most severe COPD patients; Cluster Three (3) represented obese males with sleep apnea and less severe COPD; and Cluster Four (4) represented older and comorbid patients with heart failure. These four groups demonstrated a completely different mortality pattern in the long-term follow-up, with the very severe COPD group and the older comorbid patients (Clusters 2 and 4) showing the worst survival.
Studies showing heterogeneity in the prescription of HNIV and in the profile of patients in our country19 and even in Europe,20–21 Canada and the United States22 have been published for over 20 years, independently related to the characteristics of the health care system or the economic situation in each country. Additionally, the number of patients receiving ventilatory support at home is growing,23–24 even though the evidence base for this intervention and its impact is still patchy. This substantial growth is even more evident in patients with obesity hypoventilation syndrome (OHS) and chronic obstructive pulmonary disease.25
Obesity-related disease and COPD are the two most common indications for HNIV in most surveys.26–27 A long follow-up in a HNIV cohort26 showed differential mortality patterns regarding the initial indication for this technique. In this study, patients with OHS had better survival, whereas the group with pulmonary disease (mostly represented by COPD) and motor neuron disease patients showed the worst survival. This goes in line with our results: Clusters (1) and (3) were obese males with a higher prevalence of sleep apnea that showed significantly better survival. These two groups represent two distinct phenotypes of obesity hypoventilation that coexist with COPD, and these overlapping patients form an additional phenotype in which hypoxemia and hypercapnia predominate. In a case series, the use of HNIV in these types of patients was shown to improve survival,25 and obesity is a marker of better survival in patients with COPD under HNIV.26,28 This fact, in addition to the fact that 25% of these patients were not using LTOT, seriously question whether the real indication for HNIV in these patients is obesity-related disease and not COPD.
Miravitlles et al.29 suggested that the COPD prevalence in Spain in adults aged 40–80 years was approximately 10%. According to these data, more than 400,000 people have COPD in Catalonia. Vela et al.30 estimated that 9% of these patients with COPD (more than 38,000 patients) would be in the high-risk group due to the severity of the disease, multimorbidity and social conditions. With these data, Cluster (2) (very severe COPD patients) represents a very small percentage of patients with HNIV in Catalonia (<3%) and an insignificant percentage of all high-risk COPD patients. In our study, Cluster (2) showed survival outcomes similar to those reported in several RCTs of HNIV in these patients.8,31 In addition, patients in Cluster (2) had worse lung function, the highest proportion of patients using LTOT, and the highest admissions to the ICU: all factors known to have a high impact on the survival of patients with COPD. The other cluster in our cohort with worse survival was Cluster (4), and it is totally plausible because factors such as age,26,32 comorbidities26 (including cardiovascular and heart failure) and even the use of cardiovascular agents33 have demonstrated worse survival in patients using HNIV.
This study suggests some key elements in the analysis of HNIV. Health care systems have increasingly more data available, and it makes sense (and would certainly be ethical) to use these data to understand the real world. With the available data, it is possible to study the variability in more depth and to attempt to understand the causes. With real-world data, the magnitude of the problems can be measured more accurately. Long-term ventilation of patients with severe COPD is a relatively minor problem in HNIV as a whole.
As Caneiras et al.34 showed, clinical and health resource consumption data are not enough to understand the impact of HNIV. We need to combine this information with patient experience analysis using a qualitative-quantitative approach.35
“Labels” linked to physiopathological diagnoses are not useful. The idea of clusters (groups of patients with common characteristics and needs) may be more useful for the management of patients with complex chronic diseases and many comorbidities. In this context, clusters make it possible to differentiate the cause (COPD as a cause of ventilation) from COPD as a comorbidity (which may contribute to the severity of a clinical situation). In addition, comparisons between hospitals and between territories to study variability are easier if clusters are used.
This study has several limitations. First, this was a retrospective study of COPD patients on HNIV in Catalonia. In this line, exacerbations and hospitalizations due to COPD were not recorded prospectively and were only available in the previous two years (2016–2018). Although HNIV compliance was not recorded, acceptable compliance (at least 4h) is required in daily clinical practice to continue with this treatment. However, this was a multicenter study including all COPD patients on HNIV in Catalonia. Moreover, this study included large amount of data that included reliable administrative information from the AQuAS and, along with the long-term follow-up, makes our results representative and generalizable. Further research is needed to validate the clusters identified in this study due to the small sample size.
In conclusion, in a well-characterized cohort of severe COPD patients with a long follow-up, high heterogeneity in the prescription of HNIV was demonstrated. Cluster analysis allowed us to identify different COPD patient survival groups, including an overlapping phenotype of obesity-related disease and sleep apnea that showed better survival.
Funding/supportSupported by a grant from Ramon Pla Armengol Foundation and Barcelona Respiratory Network (BRN).
Conflict of interestNo conflicts exist for any of the authors.
Author contributions: Escarrabill J and Gonzalez J are the guarantors of the paper, taking responsibility for the integrity of the work as a whole, from inception to publication. JG, PC, FB, and JE were responsible for the conception, design, interpretation, and drafting of the manuscript and provided important intellectual content. Statistical analysis was performed by EG-L. The CatCoVer group collect clinical data and contributed to correcting and improving the manuscript. We want to thanks Tomas Salas and Montse Mias from AQuAS for the support to collect the databases on which the analysis has been made.