The main function of the HLA-G molecule in its membrane-bound and soluble forms is to inhibit the immune response by acting on CD4+ T cells, cytotoxic T cells, NK cells and dendritic cells. Lung cancer is a leading cause of death worldwide, and annual incidence is high in both women and men. Some studies have reported an increase of HLA-G serum levels in lung cancer, probably generated by tumor cells escaping the antitumor immune response. In this study the concentration of soluble HLA-G in bronchoalveolar lavage (BAL) in patients with primary and metastatic lung cancer was measured to determine its relation with tumor histological type and overall patient status according to the Karnofsky scale.
MethodsThirty-one lung cancer patients were included. A tumor biopsy was obtained by bronchoscopy and the tumor type was determined by hematoxylin and eosin staining. BAL samples were obtained to measure soluble HLA-G concentrations in an ELISA sandwich assay.
ResultsThe average value of soluble HLA-G was 49.04ng/ml. No correlation between soluble HLA-G levels and age, gender or smoking was observed. A highly significant difference was observed in the levels of soluble HLA-G in BAL from patients with different histological types of lung cancer, especially in metastatic tumors. The Karnofsky index showed a significant and inverse correlation with soluble HLA-G levels in BAL.
ConclusionsSoluble HLA-G protein is significantly associated with metastatic tumors and patients with lower Karnofsky index and may be useful as a prognostic marker in lung cancer.
La molécula antígeno leucocitario humano G (HLA-G), en sus formas unida a membrana y soluble, tiene como función principal inhibir la respuesta inmune actuando sobre los linfocitos T/CD4+, T/citotóxicos, células NK y células dendríticas. El cáncer de pulmón es una de las principales causas de muerte en el mundo, con una alta tasa de incidencia anual tanto en mujeres como en hombres. Algunos estudios han reportado un incremento de HLA-G sérica en el cáncer de pulmón, probablemente como un mecanismo de escape de la célula tumoral a la respuesta inmune antitumoral. En este estudio se midió la concentración de HLA-G soluble, en el lavado broncoalveolar (LBA), en pacientes con cáncer de pulmón primario y metastásico para determinar su relación con el tipo histológico tumoral y estado general del paciente usando la escala de Karnofsky.
MétodosSe incluyeron 31 pacientes con diagnóstico de cáncer de pulmón y mediante fibrobroncoscopia se tomó biopsia de la neoplasia para determinar el tipo de tumor usando la coloración de hematoxilina y eosina, y líquido del lavado broncoalveolar para medir la concentración de la molécula HLA-G soluble mediante un ELISA tipo sándwich.
ResultadosEl valor medio de la HLA-G soluble fue de 49,04 ng/ml. No se observó ninguna correlación entre los niveles de HLA-G soluble y la edad, género o índice de tabaquismo. Se observó una diferencia altamente significativa en los niveles de HLA-G soluble en LBA de pacientes con diversos tipos histológicos de cáncer de pulmón, principalmente en tumores metastásicos. El índice de Karnofsky mostró una correlación significativa e inversa con el nivel de HLA-G soluble en LBA.
ConclusionesLa proteína HLA-G soluble puede ser útil como marcador pronóstico del cáncer pulmonar al asociarse significativamente a los tumores metastásicos y a los pacientes con menor índice de Karnofsky.
Human leukocyte antigen G (HLA-G) is a molecule which belongs to the family of proteins forming the major histocompatibility complex class I (MHC-I). Its main function is to suppress the immune response (IR), which it does by interacting with receptors expressed on the membrane of the immune system effector cells: NK cells, T cells, dendritic cells, and B cells.1–3 HLA-G is expressed in placental tissue (extravillous cytotrophoblast), where it inhibits the IR to fetal antigens, helping the pregnancy to develop.4,5 Tissues in which HLA-G has been described, either in a membrane-bound or soluble form, include the thymus, the anterior chamber of the eye, the umbilical cord, immature erythroid cells, the pancreas, and activated macrophages, among others.2 This molecule interacts with its ligands to transmit inhibitory signals to the interior of the cell which manifest as suppression of CD4+ T cell proliferation, inhibition of NK cell cytotoxicity and cytotoxic T cells, and diminished maturation of antigen-presenting dendritic cells.6–8
Lung cancer is one of the principal causes of death worldwide, originating up to 1.18 million deaths a year. Moreover, it is estimated that 900000 new cases are detected every year among men and 300000 among women, so it may be of diagnostic, therapeutic and prognostic interest to study the mechanisms leading to the development of this malignancy.9
MHC-I plays an essential role in the elimination of malignant cells by the immune system, since it helps the effector cells, NK cells and T cells to recognize the tumor cells and to infiltrate the tumor site to destroy them. However, tumor cells have developed strategies for avoiding detection and elimination by the immune system, thus promoting tumor growth; one of these strategies is the abnormal expression of immunoregulatory MHC proteins, such as HLA-G, in tumors, a response which may affect immunosurveillance.10 HLA-G expression has been detected in serum and tissue of tumors from different sources, including lung, colorectal, esophageal cancers, and B cell chronic lymphocytic leukemia, among others.11–14 Evidence shows that the HLA-G molecule, in its membrane-bound or soluble (sHLA-G) forms, determines to a large extent tumor growth and invasiveness, leading some authors to suggest its utility as a parameter in oncology.15,16
The aim of this study was to determine sHLA-G levels in bronchoalveolar lavage (BAL) samples from patients with primary or metastastic lung cancer, and to correlate the results with the histological type and patient status, using the Karnofsky performance scale.17 Thus, the direct study of sHLA-G in the tumor compartment may better reflect levels of this molecule in this disease than those obtained by measuring concentrations in serum.
Materials and MethodsPatientsAfter providing signed informed consent, 31 patients of both sexes, over 18 years of age, with primary or metastatic lung cancer, diagnosed on the basis of clinical criteria, imaging studies (chest radiograph or chest computed axial tomography), and microscopy of the lesion, attending the Instituto Autónomo «Hospital Universitario de Los Andes», in Mérida,Venezuela, were randomly selected. Patients with the following characteristics were excluded: respiratory infection at the time of admission, diffuse interstitial pulmonary disease, primary and secondary immunodeficiencies, pregnancy or recurrent abortions, contraindication for fiberoptic bronchoscopy (FB), treatment with immunosuppressive and anticancer drugs. Each patient was evaluated according to the Karnofsky performance scale. The study was approved by the local ethics committee.
Bronchoalveolar Lavage Collection and Lung Tissue Biopsy Using Fiberoptic BronchoscopyAfter applying local anesthesia, a bronchoscope was used (Olympus BF, type P10) to instill 3 aliquots of 20ml sterile saline solution at a temperature of 37°C. The saline solution was then immediately aspirated at a negative pressure of 5–120mmHg and transferred to a tube containing 1ml ethylenediaminetetraacetic acid (EDTA) for subsequent analysis. The bronchoscope was advanced to the suspicious segment (located using imaging techniques) or to the most affected segment on direct visualization, and transbronchial biopsies were obtained (4 fragments) from the affected segments or lesions. If a definitive pathological diagnosis of the cancer type could not be obtained, sampling was repeated with ultrasound-guided fine needle aspiration and biopsy, or open-sky biopsy in the operating room was scheduled, to obtain a significant, adequate sample, according to the thoracic surgeon's criteria.
Determination of the Lung Tumor Histological TypeLung tumor samples were stored in a 10% formol solution until analysis. After being removed from this solution, the tissue was embedded in paraffin and 0.3–0.4μm slices were prepared. The slices were placed on slides and stained with hematoxylin and eosin (H&E) following a protocol described elsewhere.18 The stained tissue was examined under an optical microscope (Nikon Labofhot 2, Germany) using 20× and 40× amplifications. Lesions were classified as benign or malignant, according to histological changes observed, and the cancer type was established (adenocarcinoma, epidermoid carcinoma, small cell or non-small cell cancer, and metastatic tumors).
Quantification of Levels of Soluble Human Leukocyte Antigen-G in Bronchoalveolar Lavage Using an Enzyme Immunoassay TechniqueSoluble HLA-G was quantified using a commercial assay (Human sHLA-G, elisa IBL, Germany), based on the ELISA sandwich or capture technique, following the manufacturer's specifications. Undiluted BAL samples were placed in wells in a polystyrene plate coated with a human anti-HLA-G monoclonal antibody; this antibody captures the HLA-G present in the BAL of the study subjects. A second anti-HLA-G conjugate antibody with biotin was then added and incubated for 2h at room temperature. After this time, the plates were washed 3 times with washing solution and horseradish peroxidase (HRP)-conjugated streptavidin. After incubation and 3 washings, 3,3′, 5,5′-tetramethylbenzidine (TMB) substrate was added and incubated for 10min in the dark. Finally, the stopping solution (H2SO4 2N) was added and the developed colorimetric reaction was measured using a Sunrise spectrophotometer (Tecan, Austria) with a 450nm wavelength. Values, expressed in optical densities, were transformed to ng/ml using a calibration curve and Magellan V 3.0 software.
Statistical AnalysisResults were grouped in double or triple entry tables, as appropriate. Means and standard deviations were calculated and the chi-squared and Duncan's post hoc tests were used to establish statistically significant differences between the variables. A P-value <.05 was considered statistically significant.
A general or generalized linear model (GLM) using a fixed-effect design was constructed to compare and determine the significance of sHLA-G levels among patients with different types of lung cancer.19 The factors sex and smoking index were also added to the model as fixed effects, while the effect of age on sHLA-G values was estimated as a covariate. The same GLM model was used to estimate the correlation between the Karnofsky index and sHLA-G levels in BAL.
ResultsSoluble Human Leukocyte Antigen-G Levels in Bronchoalveolar Lavage of Lung Cancer Patients are not Associated with Age, Sex, or Symptoms of DiseaseThe average age of the study population at the time of diagnosis was 59.2 years. A trend toward a higher rate of lung cancer among women than among men was observed (ratio men:women=1.22). Only 26% of the study patients reported that they were non-smokers. Finally, sHLA-G levels in BAL did not show statistically significant differences when compared with smoking index (mild, moderate or severe), age, sex and symptoms (Table 1).
General Characteristics of Patients (n=31).
| Variable | Values | sHLA-G |
|---|---|---|
| Age (years) | 59.2 (SD: ±11) | NS |
| Sex (%) | NS | |
| F | 17 (54.8) | |
| M | 14 (45.2) | |
| Smoking index (%): | NS | |
| Severe | 58.1 | |
| Moderate | 3.2 | |
| Mild | 13 | |
| Non-smoker (%) | 26 | NS |
| Pack-years | 29.6 (SD: ±36.2) | NS |
| sHLA-G in BAL (ng/ml) | 49 (SD: ±27.4) |
F, female; M, male; NS, not significant; SD, standard deviation; sHLA-G, soluble human leukocyte antigen type G.
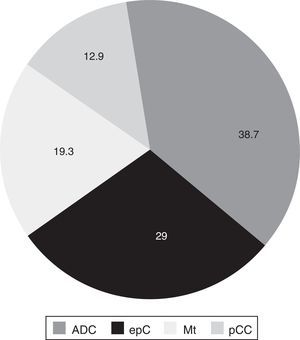
Tumor tissue was obtained mainly by FB (87.1% of samples), followed by thoractomy or open-sky biopsy (6.4%) and ultrasound guided transthoracic biopsy (6.4%). A total of 38.7% of the tumors located in the lung were adenocarcinoma, followed by epidermoid carcinoma, metastatic tumors and small-cell cancer, respectively (Fig. 1).
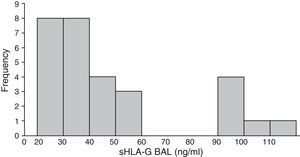
Human Leukocyte Antigen-G Occurs in Variable Concentrations in Bronchoalveolar Lavage from Lung TumorsLevels of sHLA-G in the lung compartment fluctuated widely, from a minimum concentration of 22ng/ml to a maximum of 115ng/ml, with a mean value for the overall group of 49.04ng/ml (SD±27.4ng/ml). The distribution of these levels was asymmetric and clearly bimodal. The first group (80% of patients) had sHLA-G values between 22 and 60ng/ml, and the second group had levels between 80 and 115ng/ml. All patients with metastatic tumors had sHLA-G levels of at least 90ng/ml, with an average of 98.1ng/ml (Fig. 2).
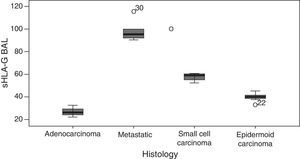
The GLM showed highly significant differences between sHLA-G levels and the tumor histological type (P<.001). Firstly, significantly higher levels of sHLA-G were found in metastatic tumors compared to primary tumors of the lung. Secondly, mean levels of this molecule were distributed as follows: adenocarcinoma had the lowest sHLA-G levels determined in the study, with a mean of 26.49ng/ml, followed by epidermoid carcinoma (mean 40.03ng/ml) and small cell carcinoma (57.60ng/ml), with metastatic lung tumors showing the highest arithmetic mean (Fig. 3).
A Correlation Between Soluble Human Leukocyte Antigen-G Levels and Patients’ Functional Status was DeterminedTo study the correlation between sHLA-G levels in BAL and the Karnofsky performance index, sHLA-G values were grouped into 2 categories: moderately high sHLA-G ≤50ng/ml, and very high >50ng/ml. These 2 categories were compared with the Karnofsky index, and a significant, inverse relationship was identified between sHLA-G levels and the Karnofsky score (P<.05). Patients with very high sHLA-G values had lower Karnofsky indices and vice versa (Table 2).
Levels of Soluble Human Leukocyte Antigen-G in Lung Cancer and Correlation with Karnofsky Index.
| Karnofsky index | sHLA-G in LBA | |||
|---|---|---|---|---|
| High (≤50ng/ml) | Very High (>50ng/ml) | Total | ||
| K | 20 | 0 (0) | 1 (10) | 1 |
| I | 30 | 0 (0) | 1 (10) | 1 |
| L | 40 | 0 (0) | 2 (20) | 2 |
| 50 | 5 (23.8) | 3 (30) | 8 | |
| K | 60 | 5 (23.8) | 3 (30) | 8 |
| I | 70 | 2 (9.5) | 0 (0) | 2 |
| H | 80 | 9 (42.9) | 0 (0) | 9 |
| Total | 21 | 10 | 31 | |
BAL, bronchoalveolar lavage; KI-H, Karnofsky index high; KI-L, Karnofsky index low; sHLA-G, soluble human leukocyte antigen-G.
Percentages are expressed in brackets.
Cancer is a major cause of morbidity and mortality throughout the world, and considerable effort is being made to identify additional diagnostic and prognostic markers which will be of benefit in the initial stages of the disease when anticancer treatment is the most effective. For this reason, some studies have indicated that serum/plasma concentrations of the molecule sHLA-G seem to be related with different tumor types, and may be a useful biomarker.15
In this study, we quantified sHLA-G levels in patients with lung cancer using BAL instead of serum/plasma. We found that this molecule is present in differing concentrations in the tumor microenvironment, and that it also appears to be directly related with the tumor histology type, since the lowest mean values were recorded for adenocarcinoma, followed by epidermoid carcinoma and small cell lung cancer. The highest values corresponded to metastatic tumors. The tumor invasion phenomenon probably requires higher levels of immunosuppression, and for this reason, metastatic lung cancers produce greater amounts of sHLA-G. This molecule is known to inhibit the antitumoral immune response by binding with the ILT-2, ILT-4 and KIR2DL4 immunoreceptors expressed in the effector cells of the immune system.2 In the case of the soluble form of HLA-G, this inhibition occurs even before contact between the tumor cell and the lymphocyte, thus facilitating tumor invasion.
Measuring sHLA-G in serum/plasma has some disadvantages. Firstly, this molecule is detected in the blood of normal subjects, at levels ranging widely between 4.40 and 14ng/ml, depending on the study.20,21 Secondly, some physiological situations, such as pregnancy and organ transplantation, cause sHLA-G levels to rise. Finally, a number of benign diseases (viral infection, asthma, and autoimmune diseases) also modify sHLA-G in serum.4,22–27 These confounding variables, which may occur in patients with lung cancer, can make it difficult to quantify sHLA-G produced exclusively by the tumor, and to determine how it impacts on the lung cancer diagnosis and prognosis. Measuring sHLA-G directly in BAL could eliminate these factors and enhance the sensitivity and specificity of this molecule as a marker of malignant lung disease, a strategy that has also been explored in the context of malignant ascites in ovarian cancer.28 The disadvantage is that samples must be obtained by invasive methods, such as FB. However, this is routinely performed for precise biopsy of the tumor and for histopathological study in most patients with suspected lung cancer. Obtaining BAL in this group, then, would not be considered an additional procedure. Other methods are useful in locating HLA-G, such as mRNA detection in tumor cells or the identification of the membrane-bound isoform by immunohistochemistry techniques.11
The Karnofsky scale has been used to evaluate the performance status of cancer patients and its impact on survival and risk of mortality; the scale has also been used to evaluate quality of life in other areas, such as gerontology.17,29 In this study we found a significant correlation between lower scores on the Karnofsky scale and higher levels of sHLA-G in BAL. The aforementioned immunosuppressive action of this molecule may affect the patient's general status, quality of life and survival prognosis by facilitating the appearance of pneumonia or the reactivation of underlying diseases. The sequence of events set in motion by locally elevated HLA-G levels and immunosuppression with pulmonary superinfection may be reflected in a reduction in Karnofsky score and a greater risk of death. Designing treatments which could correct HLA-G overproduction in the tumor microenvironment may interrupt this sequence and rescue the antitumor immune response. HLA-G is not a specific antigen against lung cancers, since it occurs in a range of tumors − breast, colorectal, esophageal, etc. − but used alongside the Karnofsky scale, it might be a prognostic marker and a determinant of the patient's clinical status.
Although more studies are needed to confirm our findings, it is of interest to continue to evaluate the benefit of sHLA-G and other similar molecules, such as HLA-E and HLA-F, as prognostic markers in patients with malignant lung disease.
Conflicts of InterestThe authors state that they have no conflict of interests.
Please cite this article as: Montilla D, Pérez M, Borges L, Bianchi G, Cova J-A. Antígeno leucocitario humano-G soluble en el lavado broncoalveolar de pacientes con cáncer pulmonar. Arch Bronconeumol. 2016;52:420–424.