Around 40% of the world's population continue using solid fuel, including wood, for cooking or heating their homes. Chronic exposure to wood smoke is a risk factor for developing chronic obstructive pulmonary disease (COPD). In some regions of the world, this can be a more important cause of COPD than exposure to tobacco smoke from cigarettes.
Significant differences between COPD associated with wood smoke (W-COPD) and that caused by smoking (S-COPD) have led some authors to suggest that W-COPD should be considered a new COPD phenotype. We present a review of the differences between W-COPD and S-COPD. On the premise that wood smoke and tobacco smoke are not the same and the physiopathological mechanisms they induce may differ, we have analyzed whether W-COPD can be considered as another COPD phenotype or a distinct nosological entity.
Alrededor del 40% de la población mundial sigue utilizando combustibles sólidos, entre ellos la leña, para cocinar o calentar sus hogares. La exposición crónica al humo de leña es un factor de riesgo para el desarrollo de enfermedad pulmonar obstructiva crónica (EPOC). En algunas zonas del mundo este factor puede ser más importante que la exposición al humo de tabaco, generalmente inhalado como humo de cigarrillo, como causa de EPOC.
Se han descrito diferencias significativas entre la EPOC relacionada con humo de leña (EPOC-L) y la EPOC causada por humo de tabaco (EPOC-T) que han llevado a plantear por algunos autores que la EPOC-L pueda ser considerada un nuevo fenotipo de la EPOC. Presentamos una revisión de las diferencias entre la EPOC-L y la EPOC-T. Basados en que el humo de la leña y el humo del tabaco no son iguales, y que podrían inducir mecanismos fisiopatológicos en algún punto diferentes, hacemos un análisis acerca de si la EPOC-L debe considerarse un fenotipo diferente de la EPOC o una entidad nosológica distinta.
A phenotype is a set of observable characteristics in an individual resulting from the interaction between their genotype and the environment.1,2 These characteristics are not only physical traits but also biochemical and functional characteristics. Genotype refers to an individual's genetic make-up (combination of genes). The manner in which the information contained in the genes (genotype) translates to observable characteristics (phenotype) depends on different factors, the most important of which are how dominant the gene is and how it interacts with the environment.1,2
In the clinical setting, the concept of phenotype has been used to identify groups of patients who share attributes that distinguish them from others, making up clinical subgroups.3 A good example of this idea of phenotype is chronic obstructive pulmonary disease (COPD), a disease in which the underlying genes are unknown (with the exception of alpha-1 antitrypsin deficiency). Since phenotype differentiation has clinical implications, the term “clinical phenotype” has been proposed, which is defined as “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death).”4
The term COPD came into use around 50 years ago5 and initially included 2 entities, chronic bronchitis and emphysema, which shared a common risk factor (smoking) and a common functional change (persistent airflow obstruction).6,7 The most pure cases of these 2 entities had sufficiently different clinical characteristics to enable them to be separated into the 2 classic COPD phenotypes: chronic bronchitis or “blue bloater” and emphysema or “pink puffer”. However, it was not known then that different phenotypes would eventually determine different therapeutic interventions and outcomes, so little importance was given to the separation of the phenotypes, and use of the generic term COPD expanded.
The concept of clinical phenotypes in COPD is now being revisited, thanks to long-term follow-up of patient cohorts and technological advances.3,4,8,9 Although the implications of separation by phenotypes are still debated, they have been included in some clinical guidelines.10 The most widely accepted phenotypes are: emphysema, chronic bronchitis, frequent exacerbator, and asthma-COPD overlap syndrome.3,4,8–10
The growing wealth of data on the differences between biomass smoke-related COPD, particularly wood smoke, and tobacco smoke-related COPD11,12 has led experts to propose biomass COPD as an additional phenotype.13,14 This proposal is controversial, and warrants a review of the existing information on these differences and the applicability of the term phenotype in the presence of risk factors that could be considered different.
In this review, we discuss the differences between wood smoke-related COPD (W-COPD) and tobacco smoke-related COPD (T-COPD). We have used the general term T-COPD, although a more accurate name would be cigarette smoke COPD, as this smoke contains an additional number of chemical products apart from those derived from burning tobacco.15,16 Since the role of these chemicals cannot be clearly separated from the role of tobacco in the pathogenesis of COPD, we will use the generic term T-COPD.
For this review, we searched the Medline, LILACS and Cochrane databases, using the terms biomass, biomass fuels, wood, wood smoke, indoor air pollution, respiratory diseases, chronic bronchitis and chronic obstructive pulmonary disease, and the connectors AND/OR.
Exposure to Wood Smoke as a Risk Factor for Chronic Obstructive Pulmonary DiseaseAround 40% of the world's population, particularly in developing countries, still use solid fuel, whether coal or biomass (wood, vegetable remains and dung), to cook or heat their homes.17,18 In some countries, these fuels are the main source of energy for over 70% of the rural population.17,18 In countries where migration from rural areas to cities is high, the population of urban dwellers over the age of 40 years frequently has a significant history of exposure to biomass combustibles. One example is Colombia, where 39% of the population over 40 years of age living in the 5 main cities had cooked with wood for more than 10 years before relocating.19 In 2010, indoor air pollution from solid fuels was the third risk factor for death throughout the world (3.5 million deaths a year).20
A growing number of studies support the hypothesis that exposure to solid fuels, including wood, is a risk factor for respiratory diseases, including acute respiratory disease in children, COPD, chronic bronchitis, airflow obstruction, bronchial hyperreactivity, asthma, tuberculosis and lung cancer.21–39 Our group has documented the association between exposure to wood smoke for over 10 years and asthma in the population >40 years of age.39
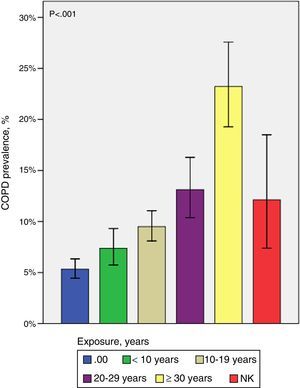
Three systematic reviews and meta-analyses confirm that individuals chronically exposed to solid fuels at home have a higher risk of developing COPD.36–38 In the case of wood smoke, the risk of COPD increases significantly with the length of exposure (Fig. 1)40 and with simultaneous exposure to tobacco smoke.40,41 Although the risk is consistently greater in women, a populational study (n=5539) showed that, after adjusting for age, smoking, educational level and occupational exposure, men exposed to wood smoke for more than 10 years had a higher risk of COPD (odds ratio [OR] women: 1.84; OR men: 1.53).40 Exposure to wood smoke has also been described as a risk factor for COPD in developed countries.33
Prevalence of COPD by years of exposure to wood smoke.40 The prevalence of COPD in individuals exposed to wood smoke increases significantly as the duration of exposure lengthens. NK: not known (individuals exposed to wood smoke who did not report years of exposure).
Air pollution in the home due to burning solid fuels is thought to be the main worldwide risk factor for COPD,42,43 although the prevalence of biomass-related COPD has not been precisely defined. The PREPOCOL study found a prevalence of 6.7% for W-COPD compared to 7.8% for T-COPD.40
Some populational studies, however, found no association between exposure to biomass fuels and COPD.44,45 Most of the cohorts evaluated in these studies lived near sea level, where cooking is usually done outdoors or with better ventilation. In contrast, many of the studies which document this association were performed in areas situated at high or intermediary altitudes, where, due to low temperatures, cooking is done all year round inside poorly ventilated homes as it occurs in winter in regions that have seasons.
Although exposure to wood smoke has been associated with respiratory diseases other than COPD,24,46–48 this review focuses on the differences between W-COPD and T-COPD.
Differences Between Chronic Obstructive Pulmonary Disease due to Wood Smoke and Chronic Obstructive Pulmonary Disease due to Tobacco SmokeAlthough the risk of COPD has been proven for all types of solid fuels, studies which best characterize COPD due to this type of exposure have focused on COPD caused by inhalation of wood smoke.11–14,24,32,34,47,49 Several studies show that W-COPD has both significant differences and similarities with T-COPD.13,40,47,49–62 The main differences are described below and summarized in Table 1.
Differences Between W-COPD and T-COPD.
| Characteristics | W-COPD | T-COPD |
|---|---|---|
| Demographic data40,47,49–51,54–56 | ||
| Sex | Predominantly women | Predominantly men |
| Age | Highest | Lowest |
| Height | Lowest | Highest |
| BMI | Highest | Lowest |
| Clinical characteristics38,49 | ||
| Cough and expectoration | Very common | Common |
| Chronic bronchitis | Common | Common |
| Rhonchus and wheezing | Common | Less common |
| Lung function tests13,40,47,49–51,54–56 | ||
| PaCO2 | Higher (some studies) | Less high |
| PaO2 and SaO2 | Lower | Less low |
| Obstruction (FEV1−FEV1/FVC) | Mild | More severe |
| Reduced FEV1 | Lowest | Highest |
| Bronchial hyperreactivity | Highest | Lowest |
| DLCO and DLCO/VA | Normal or mildly reduced | More reduced |
| Radiography-tomography13,47,49,51,54,59 | ||
| Emphysema | Uncommon and mild | Common and more severe |
| Bronchial thickening | Common | Less common |
| Bronchiectasis | Common | Uncommon |
| Atelectasis | Common | Uncommon |
| Histology | ||
| Emphysema | Mild | More severe |
| Anthracosis | Common | Less common |
| Airway fibrosis | Common | Less common |
| Thickening of arteriole intima | Common | Less common |
| Outcomes and clinical phenotypes13,51,56,61,62 | ||
| Pulmonary hypertension | More common | Less common |
| Quality of life | Similar or symptoms and activities more compromised | Similar or symptoms and activities less compromised |
| Survival | Similar after adjusting for age Less after adjusting for age | Similar |
| Exacerbator phenotype | Similar | Similar |
| Asthma-COPD overlap phenotype | More common | Less common |
| Emphysema phenotype | Uncommon | More common |
BMI: body mass index; DLCO: carbon monoxide diffusing capacity; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; PaCO2: carbon dioxide arterial pressure; PaO2: oxygen arterial pressure; SaO2: oxygen saturation; VA: alveolar volume.
W-COPD is more common in women, who are more often involved in the task of preparing food.11 Women with W-COPD are consistently reported to be shorter in height, with a higher body mass index (BMI) than women with T-COPD.40,47,49–51,54–56 Since most women with W-COPD are of a rural origin, and most of those with T-COPD are from urban conglomerations, differences in height and BMI may be due to ethnic and environmental reasons that require investigation. Moreover, women with W-COPD are older, suggesting that patients with this type of exposure need more time to develop the disease or are diagnosed later.40,49–51,55,56
Clinical DifferencesAlthough several studies have shown that the frequency of respiratory symptoms (cough, expectoration, and dyspnea) and chronic bronchitis is high in subjects exposed to biomass smoke,36,38 studies comparing W-COPD and T-COPD do not consistently find significant differences. Some studies show that W-COPD symptoms are more frequent or have more impact13,49,62 but others do not.51,53,56 With regard to the physical examination, González-García et al.49 found more frequent rhonchus and wheezing in W-COPD. Functional and tomographic findings, described below, document greater bronchial compromise, backing up studies which show more frequent cough, expectoration, rhonchus and wheezing in W-COPD.
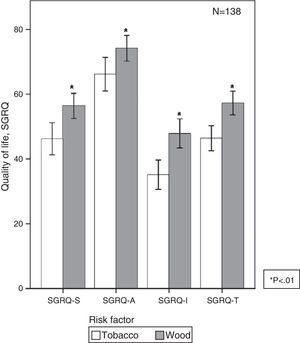
Differences in Quality of LifeA study of 138 women with COPD showed that, among women with the same degree of obstruction, those with W-COPD had a poorer health status (poorer quality of life and worse dyspnea) than those with T-COPD, with no differences in comorbidities (Fig. 2).62 Furthermore, Camp et al., using the Saint George's Hospital Questionnaire, found worse symptoms and more compromised activity indices in women with W-COPD.13
Quality of life in COPD by wood smoke or tobacco smoke exposure.62 In W-COPD, overall quality of life scores (SGRQ) and all individual domain scores are poorer. SGRQ: Saint George's Hospital Respiratory Questionnaire.
Compared with T-COPD, obstruction in W-COPD is milder, both overall and after adjusting for age,13,40,47,49–51,56 and the decline in forced expiratory volume in 1 second (FEV1) is smaller and more homogeneous than in T-COPD.50 Some studies show that carbon dioxide arterial pressure (PaCO2) is higher (lower ventilation) and oxygen arterial pressure (PaO2) and oxygen arterial saturation (SaO2) are lower in W-COPD than in T-COPD.13,49,50,56 The lower oxygenation rates observed in W-COPD may be explained in part by hypoventilation. It remains to be determined whether this behavior is related with a higher BMI in these patients, most of whom are women over 50 years of age.
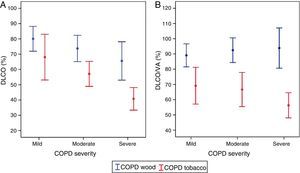
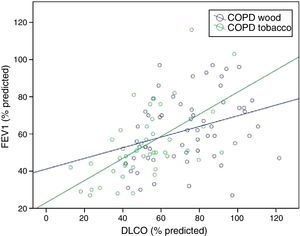
Normal or mildly altered diffusing capacity (DLCO) and DLCO/alveolar volume (DLCO/VA) ratio are consistently observed in W-COPD compared to T-COPD, in which these parameters are significantly reduced.49,54 This finding correlates with the lower grade of emphysema found on computed tomography (CT) in patients with W-COPD,13,54,59 and occurs at all levels of COPD severity (Fig. 3A and B).49 Mildly reduced DLCO with normal DLCO/VA in women with W-COPD has been described in cases with significantly compromised small airways with little emphysema (pseudophysiological emphysema).63 Compromised diffusion correlates better with reduced FEV1 in women with T-COPD than in those with W-COPD, underpinning the greater contribution of emphysema to airflow obstruction in T-COPD (Fig. 4).49
Correlation between FEV1 (%) and DLCO by exposure.49 Greater correlation is observed between FEV1 and DLCO in T-COPD (P<.001, r=0.599) than in W-COPD (P=.014, r=0.320). DLCO: carbon monoxide diffusing capacity; FEV1: forced expiratory volume in 1s.
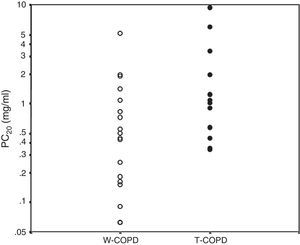
Women with W-COPD have greater bronchial hyperreactivity than women with T-COPD (Fig. 5).55 Further research is needed to determine if this correlates with the higher frequency of the asthma-COPD overlap phenotype observed in biomass-related COPD.5 Taking into account the predominant role that inhaled corticosteroids may have in patients with asthma-COPD overlap syndrome,9,10 these medications can be presumed to have a different impact on W-COPD than on T-COPD.
Bronchial hyperreactivity evaluated by PC20 by exposure.55 White circles: W-COPD; black circles: T-COPD. PC20 geometric mean: W-COPD versus T-COPD: 0.39 (0.06–5.13) versus 1.24 (0.34–9.39), P=.028. PC20: methacholine concentration causing ≥20% reduction in FEV1.
Some studies which included the 6-minute walking test found no significant differences in distances walked between patients with W-COPD and T-COPD.13,56,62 Camp et al.13 reported lower arterial oxygen saturation measured by pulse oximetry (SpO2) at the end of the test in women with W-COPD, but this was not reported in other studies.
Tomography and Histological DifferencesPatients with W-COPD have consistently less emphysema and more airway changes (bronchial thickening and fibrosis, bronchiectasis, and atelectasis) than patients with T-COPD on both chest radiographs and histological studies.13,51,52,54,59 These morphological differences can be related with a less compromised DLCO and probably with the findings of greater bronchial hyperreactivity55 and more frequent asthma phenotype in W-COPD.51
Differences in Pulmonary HypertensionA recent study found that pulmonary hypertension on echocardiography was more common in patients with W-COPD than in those with T-COPD.60 In a previous study, our group, on the basis of radiographical evaluations, suggested the same in patients with severe COPD,49 and Sandoval et al.61 showed a high rate of PH among individuals exposed to wood smoke compared to those with T-COPD. The origin of PH in W-COPD patients does not appear to be related solely with hypoxic pulmonary vasoconstriction, but also to direct effects caused by the inhaled substances or indirect inflammatory-mediated effects.64
Differences in the Incidence of Bronchial AnthracofibrosisThe incidence of bronchial anthracofibrosis and its severity in individuals exposed to wood smoke or tobacco smoke has not been evaluated in prospective studies, and no differences are known. However, anthracofibrosis is commonly encountered in the airway of subjects exposed to wood smoke, sometimes accompanied by bronchial stenosis.65–67 A significant proportion of these patients show documented airway obstruction65 possibly aggravated by their central airway stenosis. It is currently impossible to ascertain if bronchial anthracofibrosis is yet another feature of W-COPD that appears more commonly and in a more severe form than in T-COPD, or if it is a specific entity accompanied by obstruction.
Differences in Meaningful Clinical OutcomesAfter adjusting for age, sex, and disease severity, no differences were found in survival between W-COPD and T-COPD.56,57 Nor were differences identified in exacerbation rates between the 2 groups,51 but it should be noted that no prospective data are available on this aspect.
Differences in the Distribution of Clinical PhenotypesGolpe et al.51 evaluated the frequency of clinical phenotypes defined by the Spanish COPD guidelines10 in patients with COPD caused by biomass or tobacco smoke. Similarly to the findings discussed above, they found a greater frequency of emphysema phenotype in T-COPD. The asthma-COPD overlap phenotype was more common in biomass COPD, but the difference disappeared after adjusting for sex. No difference was found in the frequencies of chronic bronchitis or exacerbator phenotypes.51
Possible Reasons for Differences Between Chronic Obstructive Pulmonary Disease due to Wood Smoke and Chronic Obstructive Pulmonary Disease due to Tobacco SmokeIt is reasonable to expect that the greater airway inflammatory involvement and the lower rate of emphysema in W-COPD compared to T-COPD have an etiological, pathogenic and physiopathological basis. However, very little data are available to explain the reasons for these differences.
The composition of wood smoke, which contains hundreds of chemical compounds and particulates) is just as complex23,68 as that of cigarette smoke.15,16 Wood combustion is generally incomplete, generating greater concentrations of certain substances such as CO, benzene, and polycyclic hydrocarbons, such as benzopyrene, compared to cigarette smoke.23 Practically 100% of the particulated material in cigarette smoke is less than 2.5μm in size.69 This proportion is nearer 90% in wood smoke68; the remaining 10% of particles are between 2.5 and 10μm in size. The role of this distribution of particle size in the greater airway compromise and more common development of anthracofibrosis in W-COPD has not been determined.
Silva et al.70 recently reviewed pathogenic mechanisms involved in biomass COPD. As in T-COPD, many of these mechanisms are related with inflammatory activation and oxidative stress, whereas no obvious significant differences in the mechanisms involved in the generation of respiratory injury in W-COPD were identified. Although the lower rate or absence of emphysema in W-COPD might suggest less proteolytic activity against exposure to biomass smoke, a recent study found no differences in this respect when comparing exposure to biomass smoke and to cigarette smoke.64 Some authors have suggested that differences between W-COPD and T-COPD may be determined in part by differences in the characteristics of exposure,11 but research is needed to support this view.
In summary, the physiopathological mechanisms involved in W-COPD remain unclear, but it seems that inflammatory activation in the airway is different, and of a greater magnitude, and that proteolytic activity induces less emphysema.
Chronic Obstructive Pulmonary Disease due to Wood Smoke: A New Chronic Obstructive Pulmonary Disease Phenotype or a Different Entity?The evidence consistently supports differences between W-COPD and T-COPD with respect to greater inflammatory airway compromise and a much lower or absent degree of emphysema in the former. The etiological factors, wood smoke and cigarette smoke, can be grouped under the heading of noxious particles or gases, but they are also different,23 so it is reasonable to propose that W-COPD be considered a distinct disease, rather than a new COPD phenotype.13,14 Additionally, recognition that exposure to wood smoke may be associated with radiological, functional and histological manifestations that differ from those described under the definition of COPD, such as pulmonary infiltrates, restrictive patterns and particulate deposits in the lung,24,46–48 may be taken as yet another argument for separating it into a different nosological entity.
Even if it can be agreed, using the present definition, to describe obstructive disease caused by exposure to wood smoke as COPD, W-COPD could still not be considered a different clinical phenotype, since its clinical, functional, histological and radiological differences do not lead to differences in meaningful outcomes, such as exacerbations and mortality, as proposed in the definition of clinical phenotype.4
From a nosological point of view, the model of respiratory disease caused by wood smoke leads us to question the definition of COPD.71 COPD has been defined as a non-specific functional characteristic (irreversible airflow limitation [post-bronchodilator obstruction]) in the presence of an imprecise exposure (noxious particles or gases), in the absence of a single etiological factor or a highly specific or pathognomonic feature.72
If a single etiological factor or a highly-specific defining pathological trait is present, the identification of clinical phenotypes is a valid approach to achieve the goal of designing individualized patient management.3,9 If the etiological factor of a disease is not identified, or is not unique, as in the case of COPD (noxious particles or gases), and the disease is defined by a non-specific trait, the different characteristics and outcomes of a group may represent a distinct, separate entity, rather than another phenotype expression.
Irrespective of whether W-COPD is considered a new phenotype or a distinct entity, the most important consideration is how it affects prognosis and treatment. It can be presumed that the physiopathological mechanisms of W-COPD are different, and that a different approach to treatment may needed. In view of the predominating airway compromise, anti-inflammatories, such as inhaled corticosteroids, can be expected to play a more important role. Further research is required on the physiopathological mechanisms and treatment of disease caused by wood smoke. Better understanding of these differences could be applied to the considerable number of cases of COPD unrelated with cigarette or wood smoke,73,44 and disorders due to occupational and environmental air pollution which are classified under COPD could be better characterized.
The term COPD has been unquestionably important. On the road toward personalized treatment, beyond the stage in which phenotyping has a central role, differentiation by etiological or causative agent remains essential.71,74 The idea of COPD as a syndrome which encompasses diverse specific entities is growing, and it appears to be time to rethink the definition of COPD.3
ConclusionsW-COPD differs from T-COPD. The causative factor (wood smoke) and the characteristics of exposure are also different, and this might mean that the physiopathological mechanisms and/or its severity differ in some respect, explaining the greater inflammatory airway compromise and the lack of emphysema that are features of W-COPD. Therapeutic options would therefore also differ, and a greater role would be given to anti-inflammatories, such as inhaled corticosteroids. From this standpoint, W-COPD is better understood as a distinct disease rather than a COPD phenotype, bringing into question the accuracy of COPD definitions.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Torres-Duque CA, García-Rodriguez MC, González-García M. Enfermedad pulmonar obstructiva crónica por humo de leña: ¿un fenotipo diferente o una entidad distinta?. Arch Bronconeumol. 2016;52:425–431.