Asthma is characterized by chronic inflammation of the central and distal airways. The aim of this study was to assess the small airway (SA) of children with moderate-severe asthma with normal FEV1.
MethodsThis was an open-label, prospective, observational, cross-sectional study with consecutive inclusion of patients with moderate-severe asthma, receiving standard clinical treatment, with normal baseline FEV1. We determined multiflow FEno (CAno), oscillatory resistance and reactance (R5–R20, X5), forced spirometry (FEV1, FEF25–75), total body plethysmography (RV/TLC) and bronchodilation test. SA involvement was defined as: CAno >4.5ppb, R5–R20 >0.147kPa/L/s, X5 <−0.18kPa/L, FEF25–75 <−1.65 z-score, RV/TLC >33%. Poor asthma control was defined as ≤19 points on the ACT questionnaire or ≤20 on the c-ACT.
ResultsIn a cohort of 100 cases, 76 had moderate asthma and 24 had severe asthma; 71 children were classified as poorly controlled and 29 were well-controlled. In total, 77.78% of the group with all the correct determinations (n=72) showed ≥ 1 altered SA parameter and 48.61% ≥ 2 parameters. There were no differences between well-controlled or poorly controlled cases.
ConclusionsChildren with moderate-severe asthma, with normal FEV1, show a phenotype of dysfunctional SA. In our series, the evaluation of SA using the techniques described above did not provide information on disease control.
El asma se caracteriza por una inflamación crónica de las vías respiratorias centrales y distales. El objetivo de este estudio ha sido evaluar la vía aérea pequeña (VAP) en niños con asma moderada y/o grave con FEV1 normal.
MétodosEstudio abierto, prospectivo, observacional y transversal con inclusión consecutiva de casos con asma moderada o grave, bajo tratamiento clínico habitual con FEV1 basal normal. Se ha determinado la FEno a flujos múltiples (CAno), resistencias y reactancia oscilatorias (R5-R20, X5), espirometría forzada (FEV1, FEF25–75), pletismografía corporal total (RV/TLC) y prueba de broncodilatación. La afectación de la VAP se definió por: CAno>4,5ppb, R5-R20>0,147kPa/L/s, X5<−0,18kPa/L, FEF25–75<−1,65 z-score, RV/TLC>33%. El mal control de asma se definió por≤19 puntos en el cuestionario ACT o≤20 en c-ACT.
ResultadosCohorte de 100 casos, 76 con asma moderada y 24 con asma grave, 71 niños clasificados como mal controlados y 29 bien controlados. El 77,78% del grupo con todas las determinaciones correctas (n=72) mostró≥1 parámetro alterado de VAP y el 48,61%≥2 parámetros. No hubo diferencias entre los casos bien y mal controlados.
ConclusionesLos niños con asma moderada y grave, con el FEV1 preservado, muestran un fenotipo de VAP disfuncionante. En nuestra muestra, la evaluación de la VAP mediante las técnicas descritas, no aporta información sobre el control habitual de la enfermedad.
Asthma is a disease characterized by chronic airway inflammation that affects the entire respiratory tract, from the central airways to the more peripheral areas.1 Some researchers suggest that inflammation and airway remodeling of the small airways (SA) occurs in humans and experimental models,2 and that this SA involvement is associated with increased asthma symptoms and worse disease control. In childhood, peak expiratory flow 25%–75% is considered to be an indirect marker of SA function.3 Increased resistance and decreased reactance determined by oscillometry are associated with worse health status and poor disease control in children4 and in adults.5 Other studies suggest that mesoflow involvement is also associated with poorer disease control.6,7 Air trapping, determined indirectly by body plethysmography, has been associated with more severe exacerbations,8 more hospitalizations for asthma,9 and with worse health-related quality of life.10 Other authors have also reported that increased nitric oxide alveolar concentration (CAno) is associated with the presence of symptoms and worse asthma control in children.11 SA dysfunction is seen in patients with severe asthma.12,13
The main objective of this study was to evaluate SA function in children with asthma in routine clinical practice using normal forced expiratory volume in 1s (FEV1), to determine the potential usefulness of this parameter in asthma management.
MethodsThe study was approved by the Clinical Research Ethics Committee of our hospital. In all cases, informed consent was obtained from the parents (and from children over the age of 11 years) and permission for the use of data was given, in compliance with legal regulations.
This was a prospective, observational, cross-sectional study in patients aged between 7 and 15 years, diagnosed with asthma classified as moderate or severe according to GINA 2016,1 requiring Step 3 treatment or higher (inhaled glucocorticoids in association with long-acting β2-adrenergic agonists) for at least the previous 4 months, seen in the outpatient clinic of the pediatric respiratory department of a tertiary hospital. The recruitment period was from January 1 to June 30, 2015.
Exclusion criteria were: FEV1 lower than 80% of the predicted value, poor inhalation technique with core treatment, poor adherence (<80% of the prescribed doses),14 an asthma exacerbation in the last 7 days, treated with oral glucocorticoids in the last 7 days, acute respiratory infection and/or fever, and inability to collaborate with lung function tests.
In the absence of data on the prevalence of SA dysfunction in children with moderate or severe asthma, a pilot study was carried out in the first 30 cases to estimate the sample size (23 had moderate asthma and 7 severe). No differences were observed between the groups. There was no association between the variables of measurement of resistances between 5 and 20Hz (R5–R20), reactance at 5Hz (X5), CAno, residual volume and total lung capacity ratio (RV/TLC) and forced expiratory flow between 25% and 75% of forced vital capacity (FEF25–75). With the cut-off points used, parameters for SA dysfunction were as follows CAno (n=4), R5–R20 (n=13), X5 (n=12), FEF25–75 (n=3) and RV/TLC (n=6). Recruitment continued until a sufficient theoretical sample size (n=100) was reached to meet the study objective.
In all cases, the fraction of exhaled nitric oxide was determined from a single breath and multiple flows (30, 150, and 250ml/s), using the Eco Medics UNCCD 88 SP® chemiluminescence stationary analyzer with Denox 88 flow adaptors, to obtain CAno using the 2-compartment model and the equation of Tsoukias and George.15 We then performed, in the following order, pulse oscillometry (PO) (MasterLab version 5.1, Viasys®, Wuerzburg, Germany), forced spirometry (MasterScreen v. 4.67, Viasys®, Germany), and total body plethysmography (MasterLab version 5.1, Viasys®, Wuerzburg, Germany). After these baseline tests, the bronchodilation (BD) test was performed, administering inhaled salbutamol (400μg) with a Volumatic® spacer and repeating PO, forced spirometry, and plethysmography 15min later. All these techniques were performed according to the published recommendations.16–20
We used the reference values of Lechtenbörger et al.21 for PO, Jaeger22 for plethysmography resistances, and the equations proposed by Zapletal23 for forced spirometry and plethysmography. The BD test was considered positive in case of FEV1 ≥12% compared to the previous value or ≥9% compared to the predicted value.24
SA dysfunction was defined as CAno >4.5ppb25; R5–R20 >0.147kPa/L/s4; X5 <−0.18kPa/L4; FEF25–75 <65% predicted26; and/or RV/TLC >33%.27
We calculated the z-score corresponding to 65% of the predicted value of FEF25–75, using logistic regression in the sample, which was (−1.65) the value used as the cut-off point.
All subjects completed the ACT asthma control questionnaire in Spanish for patients aged over 12 years28 or the c-ACT pediatric version in the case of children under the age of 12 years.29 Poor asthma control was defined as a score of ≤19 points on the ACT questionnaire or ≤20 on the c-ACT.
Data were processed using the Stata statistical package version 14.1® (Stata Corp., TX, USA), in a specifically designed database. A P<.05 was considered statistically significant. A descriptive analysis was performed of demographic and baseline characteristics, which included the number of observations, mean and standard deviation from the mean for continuous variables, and frequencies for categorical variables. Variables with non-normal distribution were expressed as median and range, and the analysis was carried out using non-parametric statistics (Kruskal–Wallis test). For continuous variables, the differences between the 2 groups were analyzed using Student's t test for independent samples and analysis of variance, and Pearson's chi-squared test or Fisher's exact test were used for categorical variables. We analyzed the correlation and degree of agreement of the different parameters that evaluate the SA using Spearman's correlation coefficient and Cohen's kappa coefficient.
ResultsA total of 139 cases were recruited consecutively from the outpatient clinic of the pediatric respiratory department. Thirty-nine cases who met one or more of the exclusion criteria were excluded: 4 receiving bronchodilation; 1 permission not given; 11 non-collaborative for functional testing; 3 received treatment with oral glucocorticoids in the last 7 days; 3 had current respiratory infection; 8 current exacerbation; 6 poor treatment compliance; and 3 had FEV1 less than 80% predicted.
Mean age of the 100 cases included was 11.1±2.53 years (range 7–15), with a predominance of males (n=67). A total of 76 cases were classified as moderate asthma and 24 as severe asthma; 71 cases were poorly controlled and 29 well-controlled, with no differences between groups of moderate and severe asthma (P=.12).
All cases (n=100) performed all the tests and presented all variables, except for 28 cases where the determinations of the fraction of exhaled nitric oxide did not follow the linear model. There were no significant differences between moderate asthma and severe asthma in non-dependent variables of SA in the nitric oxide, spirometry and plethysmography tests, except for PO: cases with severe asthma had higher R5 (P=.02) and Z5 (P=.02) values.
For the SA variables, cases of severe asthma showed higher R5–R20 values (P=.02) (Table 1). SA parameters indicated dysfunction at the rates shown in Table 2. There were no differences between moderate asthma and severe asthma, except in FEF25–75, which was more often abnormal in severe asthma (P=.04). Of the cases with complete, valid results (n=72), 77.78% presented 1 or more abnormal parameter and 48.61% had 2 or more; no significant differences were found between moderate asthma and severe asthma (P=.27) (Table 3).
Mean Values of the Various Parameters Indicative of Airway Involvement in the Overall Group of Asthmatic Children and in Subgroups of Moderate Asthma and Severe Asthma.
| SA Parameters | Overall Group (n=100) | Moderate Asthma (n=76) | Severe Asthma (n=24) | Pa |
|---|---|---|---|---|
| CAno [ppb, median (range)] | 1.45 (0–15) | 0.6 (0–9.4) | 1.15 (0–15) | .57b |
| R5–R20 (kPa/L/s, mean±SD) | 0.14±0.11 | 0.13±0.10 | 0.19±0.14 | .02 |
| X5 (kPa/L/s, mean±SD) | −0.19±0.91 | −0.19±0.08 | −0.22±0.12 | .15 |
| FEF25–75 (z-score, mean±SD) | −0.76±1.22 | −0.68±1.13 | −1.02±1.50 | .24 |
| RV/TLC (%, mean±SD) | 28.38±6.29 | 28.34±6.37 | 28.54±6.20 | .89 |
CAno: alveolar concentration of nitric oxide; FEF25–75: forced expiratory flow between 25% and 75% of the forced vital capacity; R5–R20: resistance between 5 and 20Hz; RV/TLC: residual volume/total lung capacity ratio; SA: small airways; SD: standard deviation; X5: resistance at 5Hz; z-score: standardized variable to compare data.
Mean Values of the Various Parameters Indicating Small Airway Involvement in the Overall Group of Asthmatic Children and in Subgroups of Moderate Asthma and Severe Asthma.
| SA Involvement | P | ||||||
|---|---|---|---|---|---|---|---|
| Overall Group (n=100) | Moderate Asthma (n=76) | Severe Asthma (n=24) | |||||
| n | % | n | % | n | % | ||
| CAno (n=72) | 7 | 9.72 | 4 | 5.26 | 3 | 12.5 | .37a |
| R5–R20 (n=100) | 49 | 49 | 24 | 24 | 15 | 15 | .13b |
| X5 (n=100) | 47 | 47 | 33 | 33 | 14 | 14 | .20a |
| FEF25–75 (n=100) | 26 | 26 | 16 | 16 | 10 | 10 | .04b |
| RV/TLC (n=100) | 20 | 20 | 16 | 16 | 4 | 4 | .77a |
CAno: alveolar concentration of nitric oxide; FEF25–75: forced expiratory flow between 25% and 75% of the forced vital capacity; R5–R20: resistance between 5 and 20Hz; RV/TLC: residual volume/total lung capacity ratio; SA: small airways; X5: resistance at 5Hz.
Number of Parameters Indicating Abnormal Airway Involvement in the Overall Group of Asthmatic Children and in Subgroups of Moderate Asthma and Severe Asthma.
| No. of Abnormal Parameters | Overall Group (n=72) | Moderate Asthma (n=53) | Severe Asthma (n=19) | |||
|---|---|---|---|---|---|---|
| Cases | % | Cases | % | Cases | % | |
| 0 | 16 | 22.22 | 14 | 19.44 | 2 | 2.78 |
| 1 | 21 | 29.17 | 13 | 18.06 | 8 | 11.11 |
| 2 | 16 | 22.22 | 13 | 18.06 | 3 | 4.17 |
| 3 | 13 | 18.06 | 9 | 12.5 | 4 | 5.56 |
| 4 | 5 | 6.94 | 4 | 5.56 | 1 | 1.39 |
| 5 | 1 | 1.39 | 0 | 0 | 1 | 1.39 |
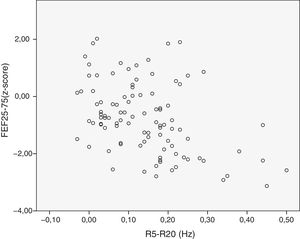
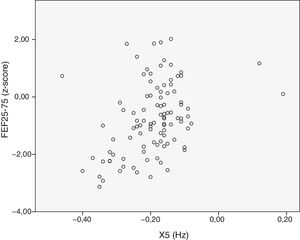
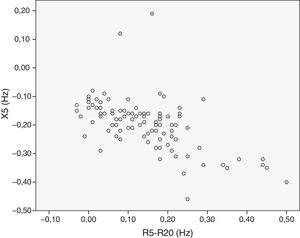
We analyzed the relationship between the dependent SA variables. There was a moderate correlation between FEF25–75 and R5–R20 (r=−0.58) (Fig. 1), between FEF25–75 and X5 (r=0.57) (Fig. 2), and between R5–R20 and X5 (r=−0.58) (Fig. 3). There was moderate agreement between R5–R20 and X5 (κ=0.44; P<.05) and low agreement between R5–R20 and FEF25–75 (κ=0.29; P<.05), X5 and FEF25–75 (κ=0.28; P=.00) and RV/TLC and FEF25–75 (κ=0.27; P<.05).
The BD test was positive in 19 cases. In this group, there were no differences between moderate asthma and severe asthma, nor among subgroups of good and poor control. Patients with a positive BD test positive obtained worse results than the group with a negative BD test (Table 4).
Comparisons of the Various Parameters Indicating Small Airway Involvement in the Group of Asthmatic Children With Positive and Negative Bronchodilation Test.
| Negative BD Test | Positive BD Test | Pb | |||
|---|---|---|---|---|---|
| n | (Mean±SD) | n | (Mean±SD) | ||
| CAno (ppb) | 59 | 1.22 (0–15)a | 13 | 3.30 (0.2–9.4)a | .00c |
| R5–R20 (kPa/L/s) | 81 | 0.13±0.10 | 19 | 0.20±0.14 | .01 |
| X5 (kPa/L) | 81 | −0.18±0.09 | 19 | −0.24±0.09 | .01 |
| FEF25–75 (z-score) | 81 | −0.49±1.11 | 19 | −1.93±0.99 | .00 |
| RV/TLC (%) | 81 | 27.88±6.25 | 19 | 30.56±6.20 | .09 |
BD: bronchodilation; CAno: alveolar concentration of nitric oxide; FEF25–75: forced expiratory flow between 25% and 75% of forced vital capacity; R5–R20: resistance between 5 and 20Hz; RV/TLC: residual volume/total lung capacity ratio; SD: standard deviation; X5: resistance at 5Hz; z-score: standardized variable to compare data.
SA parameters were compared among groups with good and poor disease control, and no statistically significant differences were found in any of the variables (Table 5). Similarly, the sum of different abnormal parameters was not indicative of poorer disease control (P=.07).
Mean Values of the Various Parameters Indicating Small Airway Involvement in the Overall Group of Children With Good and Poor Asthma Control.
| Good Control | Poor Control | pb | |||
|---|---|---|---|---|---|
| n | (Mean±SD) | n | (Mean±SD) | ||
| CAno (ppb) | 48 | 1.45 (0–15)a | 24 | 1.41 (0.1–9.4)a | 0.89c |
| R5–R20 (kPa/L/s) | 71 | 0.13±0.11 | 29 | 0.15±0.11 | 0.49 |
| X5 (kPa/L) | 71 | −0.18±0.09 | 29 | −0.21±0.08 | 0.10 |
| FEF25–75 (z-score) | 71 | −0.76±1.11 | 29 | −0.77±1.48 | 0.99 |
| RV/TLC (%) | 71 | 28.24±6.34 | 29 | 28.73±6.27 | 0.72 |
CAno: alveolar concentration of nitric oxide; FEF25–75: forced expiratory flow between 25% and 75% of forced vital capacity; R5–R20: resistance between 5 and 20Hz; RV/TLC: residual volume/total lung capacity ratio; SD: standard deviation; X5: resistance at 5Hz; z-score: standardized variable to compare data.
Finally, the number of abnormal SA parameters was not associated with hospitalizations for asthma during the past 12 months (2 cases) (Fisher's exact test, P=.06).
DiscussionThis study included patients with FEV1 higher than 80% predicted value with the aim of assessing whether SA dysfunction could be the reason for not achieving complete disease control in patients with normal proximal airway function.
One of the main problems in analyzing and evaluating the degree of SA involvement is difficulty in accessing this region, and the different techniques used have their limitations. FEF25–75 is one of the most commonly cited measurements of SA involvement, although its limitations are known. In the absence of expiratory airflow limitation, an increase in RV could be attributed to a premature closure of the SA, and given that the TLC does not usually change in asthma, SA involvement could manifest itself through an increase in the RV/TLC ratio, a marker of air trapping and pulmonary hyperinflation. In PO, the difference between total resistance and central resistance [R(5Hz)−R(20Hz)] corresponds to SA resistance, while pulmonary reactance measured at 5Hz reflects the elastic properties, capacitance, and the degree of obstruction of the peripheral airways. Moreover, CAno is interpreted as a surrogate marker of inflammatory involvement of the SA.
One of the key findings of our study was that 77.78% of children with moderate and severe asthma who performed all SA tests (72 cases), treated with a combination of medium or high-dose ICS and 2 β2-adrenergic agonists, with normal FEV1 and a very low number of hospitalizations during the last year (2 cases), had at least 1 criterion for SA involvement, as also reported by Pisi et al.30 and Wagner et al.31 in asthma patients with normal FEV1 values. This finding, which we consider of interest, warrants the conduct of longitudinal studies to evaluate its significance in the management and good control of children with asthma.32
SA involvement was evidenced primarily by PO, by both R5–R20 (n=49) and X5 (n=47). However, FEF25–75, RV/TLC and CAno showed fewer abnormal results [n=26, n=20 and n=7 (9.72%), respectively], which indicates that the cut-off points used for the PO parameters might be either too sensitive to changes in the peripheral airways or not very specific.
In terms of CAno, the 2-compartmental model of nitric oxide could not be applied in 28% of our cases. This figure is somewhat higher than reported by other authors25,33 and could be explained by differences in ventilatory and inflammatory patterns. The cut-off point of normality was set at the upper limit calculated for the group of healthy children in our population (4.5ppb),25 which is similar to that described by other authors33,34 who have used the same methodology. High concentrations of alveolar nitric oxide were not detected in our study, which does not rule out inflammatory involvement of the SA, since both CAno levels and bronchial oxide concentration can be affected by the use of inhaled glucocorticoids.35 Similarly to van Veen et al.,36 we found no differences in CAno among the groups with moderate asthma and severe asthma.
SA evaluation using PO showed significantly higher values of R5–R20 in the group of children with severe asthma (0.19±0.14Hz), a result consistent with previous publications that report SA dysfunction in severe asthma.37 No studies have been performed on dependent PO variables in the pediatric population to determine the cut-off point for SA involvement. For this reason, we used the cut-off points determined by Shi et al.4 in a study performed to determine the usefulness of PO in disease control in asthmatic children, with the limitations that this implies. With these parameters, almost half of the cohort studied showed an increase in peripheral airway resistance and reduced reactance, suggesting that while these cut-off points are very sensitive for detecting SA changes, they are not useful for discriminating between moderate and severe asthma or well and poorly controlled asthma.
The use of FEF25–75 for the study of SA is controversial, because of the dependence of this parameter on FVC and its low reproducibility.38 Our study cohort showed a mean z-score of −0.76±1.22 that is far from pathological, and no differences were observed between groups of different severity, probably due to the wide variability of this parameter. We selected a FEF25–75 cut-off point on the basis of a study carried out in 700 children with asthma designed to define the cut-off point of this parameter.26 We then calculated the z-score for our sample. In the analysis performed with this cut-off point, FEF25–75 was the only SA parameter that showed a greater frequency and proportion in the group with severe asthma (P=.04). Although FEF25–75 is the most accessible parameter in clinical practice, it should be interpreted with caution because of the limitations discussed previously.
We, like Sorkness et al.,39 chose the RV/TLC ratio as an index of hyperinflation (air trapping). In order to identify hyperinflation with a high level of confidence, we defined the cut-off value as the 95th percentile of RV/TLC in children, which is 0.33.27 In our study, mean RV/TLC was 28.38%±6.29%, and no significant differences were observed between the groups with moderate asthma and severe asthma. In our series, 20% of children had RV/TLC higher than 33%, compared to 7–11% detected by Mahut et al.,40 probably because those cases had less severe asthma.
A total of 19 cases had a positive BD test. These patients had poorer results in SA markers than patients with negative BD results, so we can conclude that some degree of proximal airway obstruction does not confound SA analysis. In the group of children with positive BD test, the dependent variables of post-BD SA on PO, spirometry and plethysmography were analyzed, and a significant improvement in all indicators of SA involvement was observed.
We analyzed the usefulness of the SA study for defining the degree of disease control, and found that none of the variables related to SA showed any association with the degree of asthma control. Similarly, the sum of the different abnormal parameters was not associated with worse disease control. This result is in line with a study in adults with moderate or severe asthma12 and normal proximal airways, in which no association was found between the ACT questionnaire score and the different SA parameters in the 222 patients studied. c-ACT and ACT appear to correlate well with the GINA criteria1 for predicting the risk of uncontrolled asthma, but the cut-off points commonly used for c-ACT and ACT appear to underestimate the proportion of children with uncontrolled asthma as defined by GINA.41 This might affect our results for the association between SA dysfunction and disease control. Another hypothesis to explain the lack of association between SA dysfunction and disease control could be that this is not a reason for poorer asthma control, but instead is a yet another feature of the disease, or else a phenomenon associated with other factors such as the allergic response,42 or disease persistence, progress or prognosis.
The main limitation of our study is the absence of reference values to determine SA involvement. Moreover, the cross-sectional design makes it impossible to study the behavior of SA over time, in a disease in which variability over time is a defining characteristic. Similarly, the heterogeneity of severe asthma makes it difficult to study, because of the variability it can display depending on the time that studies are performed. Finally, the lack of technical guidelines for determining CAno should be taken into account. We therefore need longitudinal multicenter studies to clarify these and other doubts.
ConclusionsMost children with moderate and severe asthma receiving treatment recommended for FEV1 within normal ranges have a dysfunctional SA phenotype in terms of resistance, reactance, inflammation, limitation of the peak expiratory flow or air trapping, with a moderate degree of agreement between them. This SA dysfunction is not related to the level of asthma control.
FundingThis research project has not received any funding.
Conflict of InterestsThe authors state that they have no conflict of interests.
We thank José I. Emparanza, MD, PhD, Clinical Research Unit of the Hospital Universitario de Donostia for performing the statistical analysis.
Please cite this article as: Azaldegi G, Korta J, Sardón O, Corcuera P, Pérez-Yarza EG. Disfunción de la pequeña vía aérea en niños con asma controlada. Arch Bronconeumol. 2019;55:208–213.