Although an association between uric acid (UA) levels and obstructive sleep apnea (OSA) has been reported, the effect of continuous positive airway pressure (CPAP) on this measure is yet unclear. We aimed to investigate the effect of CPAP therapy on serum UA levels in patients with OSA.
MethodsWe conducted a multicenter, open-label, randomized controlled trial in 307 women diagnosed with moderate-to-severe OSA (apnea-hypopnea index [AHI]≥15) in 19 Spanish Sleep Units. Women were randomized to CPAP (n=151) or conservative treatment (n=156) for 12 weeks. Changes in serum UA measures were assessed on an intention-to-treat basis. Additional analyses were conducted in the subgroup of women with CPAP adherence ≥4h/night and those with UA levels ≥6mg/dl.
ResultsWomen had a mean (SD) age of 57.1 (10.1) years, median (first–third quartile) body mass index of 33.7 (29.0–38.5) mg/kg2 and AHI of 32.0 (22.6–48.5). The average serum UA measure was 5.11 (1.26) mg/dl, and 80 (26.1%) participants had UA≥6mg/dl. Compared with the control group, the CPAP group did not achieve any reduction in UA levels (non-adjusted intergroup difference −0.03mg/dl, 95%CI −0.20 to 0.13; p=0.702) after 12 weeks of follow-up. These results did not change when the analysis was restricted to women with CPAP adherence ≥4h/night, or the subgroup of women with hyperuricemia.
ConclusionsTwelve weeks of CPAP therapy does not reduce UA levels compared to conservative treatment in women with moderate-to-severe OSA.
Aunque se ha determinado una asociación entre los niveles de ácido úrico (AU) y el síndrome de apnea obstructiva del sueño (SAOS), el efecto de la presión positiva continua en las vías aéreas (CPAP) en esta medida todavía no está claro. El objetivo fue determinar el efecto de la CPAP en los niveles séricos de AU en pacientes con SAOS.
MétodosSe llevó a cabo un ensayo abierto, aleatorizado, controlado, multicéntrico en 307 mujeres diagnosticadas con SAOS de moderado a grave (índice de apneas-hipopneas [IAH]≥15) en 19 unidades del sueño españolas. Fueron aleatorizadas a recibir CPAP (n=151) o tratamiento conservador (n=156) durante 12 semanas. Los cambios en las medidas de AU sérico se estimaron mediante análisis por intención de tratar. Se llevaron a cabo análisis adicionales en el subgrupo de mujeres con adherencia a CPAP≥4h/noche y en aquellas con niveles de AU≥6mg/dl.
ResultadosLa edad media (DE) de las participantes fue 57,1 (10,1) años, la mediana (primer y tercer cuartil) del índice de masa corporal 33,7 (29,0-38,5) mg/kg2 y el IAH 32,0 (22,6-48,5). El nivel medio de AU fue 5,11 (1,26) mg/dl, y 80 (26,1%) participantes tuvieron AU≥6mg/dl. Comparado con el grupo control, el grupo CPAP no consiguió ninguna reducción de los niveles de AU (diferencia intergrupo no ajustada: −0,03mg/dl; IC 95%: −0,20-0,13; p=0,702) tras 12 semanas de seguimiento. El análisis no varió cuando se restringió a las mujeres con adherencia a CPAP≥4h/noche o al subgrupo de mujeres con hiperuricemia.
ConclusionesDoce semanas de terapia con CPAP no reducen los niveles de AU en comparación con el tratamiento conservador en mujeres con SAOS de moderado a grave.
Obstructive sleep apnea (OSA) is characterized by repetitive episodes of upper airway collapse during sleep that lead to intermittent hypoxia and sleep fragmentation. These repeated cycles of hypoxia/reoxygenation are associated with increased production of reactive oxygen species, oxidative stress and systemic inflammation, which in the long-term may result in hypertension and other cardiovascular diseases.1
The intermittent hypoxia found in OSA may influence the metabolic pathway of purines, increasing adenosine triphosphate degradation and the release of purine intermediates, which eventually leads to an overproduction of uric acid (UA). In fact, hyperuricemia and increased overnight urinary UA excretion is a common finding in patients with OSA,2 and an independent association between OSA severity and UA levels has been observed in population-based and clinical cohorts.3
UA has been recognized as a potential cardiovascular risk factor. Longitudinal studies have found an independent role for serum UA in the prediction of cardiovascular morbidity and mortality,4,5 while hyperuricemia has recently been associated with cardiovascular disease in OSA patients, even after controlling for other classic risk factors.6
Very few studies have analyzed the effect of continuous positive airway pressure (CPAP) therapy on UA levels in OSA patients, and even these have presented conflicting results. Although some observational studies have reported a reduction in UA levels, the only randomized controlled trial published to date did not find any effect on this outcome, although this study was limited by its small sample size (only 39 patients).2,7,8 Thus, the aim of this study was to investigate the effect of CPAP therapy on serum UA levels in a large sample of women diagnosed with moderate-to-severe OSA, using a randomized controlled trial design.
MethodsDesign, settings, and participantsThis study is part of a larger study, designed to investigate the effect of CPAP therapy on multiple clinical outcomes, in a population exclusively composed of women with OSA (NCT02047071). The effects of CPAP on quality of life, blood pressure, and glucose and lipid metabolism have been previously reported.9,10 The present study focuses on one of the secondary endpoints: the effect on serum UA levels.
We conducted a multicenter, open-label, randomized-controlled trial of parallel groups with final blind evaluation. The participants were enrolled in 19 Spanish Sleep Units between February 2014 and February 2015. The study was approved by the Ethics Committee of each participating center and all the participants provided informed signed consent.
Women were eligible if they were aged between 18 and 75 years and had been referred for suspicion of OSA and finally diagnosed with moderate-to-severe OSA (apnea–hypopnea index [AHI]≥15). Women were excluded if they had respiratory failure, chronic kidney or liver disease, heart failure grade III–IV NYHA, unstable cardiovascular disorders, pregnancy, severe daytime sleepiness (Epworth sleepiness score [ESS]>18), prior diagnosis of OSA or CPAP treatment, or central sleep apnea (more than 50% of apneic events were central).
ProceduresSleep studyEvery woman underwent a diagnostic sleep study by means of an unattended, home respiratory polygraphy, using a device validated against polysomnography, and following the Spanish Society of Pneumology and Thoracic Surgery Guidelines for OSA diagnosis and treatment.11 Every sleep study was manually scored by skilled staff. All the studies included continuous recording of the oro-nasal flow and pressure, respiratory movements, and oxyhemoglobin saturation (SaO2). Apnea was defined as complete cessation of oro-nasal flow for ≥10s and was classified as either obstructive or central, based on the presence or absence of respiratory efforts. Hypopnea was defined as a 30–90% reduction in oro-nasal flow for ≥10s followed by a ≥3% decrease in SaO2. The AHI was defined as the number of apneas plus hypopnea per hour of recording. A sleep study was considered as valid if at least 4h of recording and more than 3h of subjective sleep were reported. Invalid studies were repeated.
Baseline visitAfter OSA diagnosis, the participants completed a standardized protocol that included general and anthropometric data, menopausal status, history of cardiovascular diseases, current medications, subjective sleep duration, subjective sleepiness measured by the ESS, and clinical history related to OSA.
Serum UA assessmentVenous blood samples were collected after an overnight fast and were drawn directly into sterile tubes containing EDTA as anticoagulant. All the samples were studied within 1–2h following blood collection, without storage. Serum UA levels were measured using standard laboratory assays (uricase method) at the baseline visit and after 12 weeks of follow-up. Glucose and lipid measures, liver enzymes, creatinine and urea levels were also assessed.
Randomization and interventionParticipants were randomized to either CPAP or conservative treatment by using a computer-generated list of random numbers in the coordinating center and stratified by center. All the women received dietary and sleep hygiene counseling. The participants were advised not to change their cardiovascular and hypouricemiant medication during the follow-up, unless this was dictated by clinical needs.
For those women randomized to CPAP therapy, the optimal pressure was titrated on a second night, using an auto CPAP device, according to a previous validation by the Spanish Sleep Network.12 The optimal pressure was determined in a centralized manner, by two blinded expert researchers, based on the visual evaluation of the raw data recording from the night study, with no significant leaks (less than 0.40L/s). This fixed pressure was maintained throughout the study.
The final visit was scheduled after 12 weeks of the CPAP onset in the treatment group, and after 12 weeks of randomization in the conservative treatment group. In this appointment, objective adherence to CPAP, side effects (for CPAP group), and changes in treatment were assessed, and fasting blood samples were taken again.
Study endpointThe endpoint addressed in the present study corresponds to one of the secondary endpoints of the larger study, namely changes in serum UA levels at 12 weeks compared to baseline, in the CPAP versus the control group.
Statistical analysisResults are expressed as mean (SD) or median (first-third quartile) for continuous variables and number of patients (%) for categorical variables. Normality in the variables distribution was assessed with the Kolmogorov test. Baseline continuous variables were compared using Student's t-test or Mann–Whitney test, and categorical variables were compared using Chi-square or Fisher exact tests.
The inter-group comparison of the changes in UA levels were assessed by analysis of covariance. The analyses were performed on an intention-to-treat basis. Additional sensitivity analyses were conducted to investigate the effect of CPAP therapy on the subgroup of women with high UA levels (baseline levels ≥6mg/dl),13 as well as on a per-protocol analysis, including in the CPAP group only those patients who used the device for at least 4h/night on average.
A sample size of 307 women (151 randomized to CPAP and 156 to conservative treatment) was calculated for the primary endpoint of the original study (quality of life),9 but also for enough power to analyze other secondary endpoints. Thus, this sample size would enable us to detect a change of at least 0.5mg/dl in UA levels between the CPAP and control group, with an α error of 0.05 and a power of 80%, considering a SD of 1.5mg/dl (at least 140 participants would be needed in each group).7,14
Missing values for the primary endpoint were imputed by baseline observation carried forward.
The statistical analyses were performed using a blind evaluation of the study groups.
Two-tailed p-values of <0.05 were considered significant. IBM SPSS 19.0 statistical package (SPSS Inc., Chicago, IL, USA) was used for data processing and analysis.
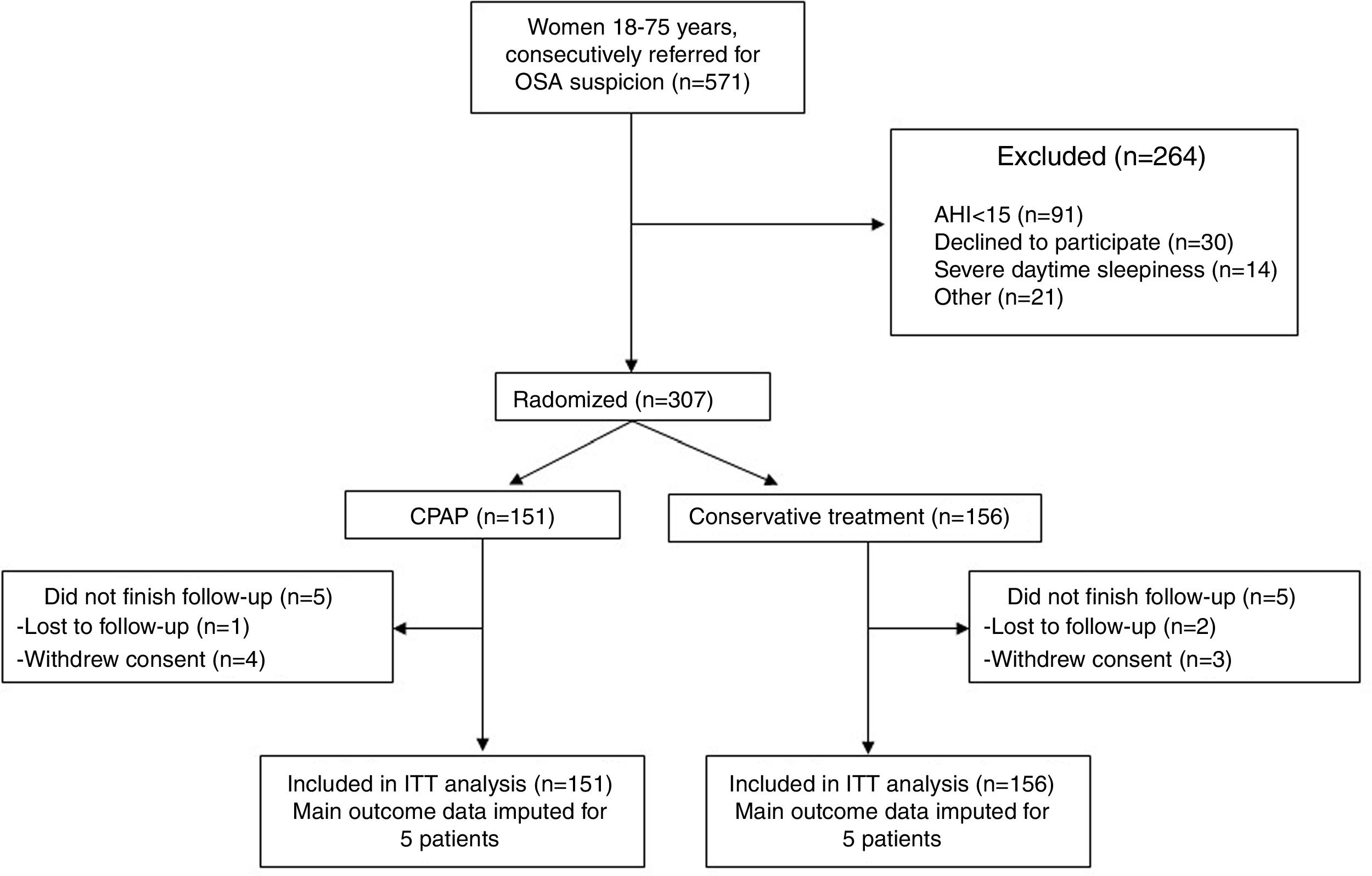
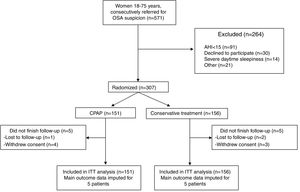
ResultsOf a total of 571 women referred for OSA suspicion, 307 were finally eligible for the study, whereas 264 were excluded (Fig. 1). One hundred and fifty-one participants were allocated to CPAP and 156 to the control group (conservative treatment). Only 5 women in each group did not complete the follow-up.
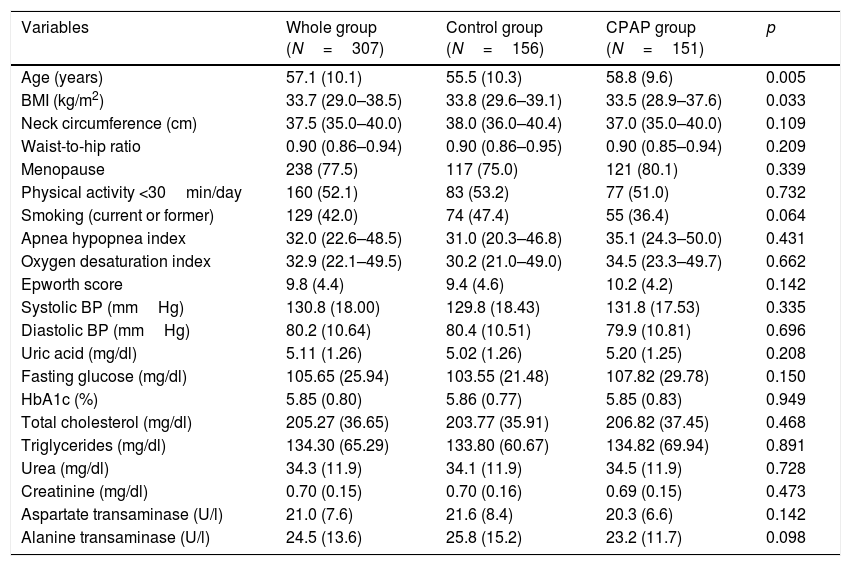
The general baseline characteristics were comparable in both groups, except for age and BMI (Table 1). Women in the CPAP group were older and slightly less obese than those in the control group, but there were no differences in other anthropometric features, baseline BP values, glucose or lipid parameters, UA levels, liver transaminases, creatinine or urea measurements between groups (Table 1). At the end of the follow-up no significant change in weight was observed in either the CPAP or control groups.
Baseline characteristics of the women included in the study.
| Variables | Whole group (N=307) | Control group (N=156) | CPAP group (N=151) | p |
|---|---|---|---|---|
| Age (years) | 57.1 (10.1) | 55.5 (10.3) | 58.8 (9.6) | 0.005 |
| BMI (kg/m2) | 33.7 (29.0–38.5) | 33.8 (29.6–39.1) | 33.5 (28.9–37.6) | 0.033 |
| Neck circumference (cm) | 37.5 (35.0–40.0) | 38.0 (36.0–40.4) | 37.0 (35.0–40.0) | 0.109 |
| Waist-to-hip ratio | 0.90 (0.86–0.94) | 0.90 (0.86–0.95) | 0.90 (0.85–0.94) | 0.209 |
| Menopause | 238 (77.5) | 117 (75.0) | 121 (80.1) | 0.339 |
| Physical activity <30min/day | 160 (52.1) | 83 (53.2) | 77 (51.0) | 0.732 |
| Smoking (current or former) | 129 (42.0) | 74 (47.4) | 55 (36.4) | 0.064 |
| Apnea hypopnea index | 32.0 (22.6–48.5) | 31.0 (20.3–46.8) | 35.1 (24.3–50.0) | 0.431 |
| Oxygen desaturation index | 32.9 (22.1–49.5) | 30.2 (21.0–49.0) | 34.5 (23.3–49.7) | 0.662 |
| Epworth score | 9.8 (4.4) | 9.4 (4.6) | 10.2 (4.2) | 0.142 |
| Systolic BP (mmHg) | 130.8 (18.00) | 129.8 (18.43) | 131.8 (17.53) | 0.335 |
| Diastolic BP (mmHg) | 80.2 (10.64) | 80.4 (10.51) | 79.9 (10.81) | 0.696 |
| Uric acid (mg/dl) | 5.11 (1.26) | 5.02 (1.26) | 5.20 (1.25) | 0.208 |
| Fasting glucose (mg/dl) | 105.65 (25.94) | 103.55 (21.48) | 107.82 (29.78) | 0.150 |
| HbA1c (%) | 5.85 (0.80) | 5.86 (0.77) | 5.85 (0.83) | 0.949 |
| Total cholesterol (mg/dl) | 205.27 (36.65) | 203.77 (35.91) | 206.82 (37.45) | 0.468 |
| Triglycerides (mg/dl) | 134.30 (65.29) | 133.80 (60.67) | 134.82 (69.94) | 0.891 |
| Urea (mg/dl) | 34.3 (11.9) | 34.1 (11.9) | 34.5 (11.9) | 0.728 |
| Creatinine (mg/dl) | 0.70 (0.15) | 0.70 (0.16) | 0.69 (0.15) | 0.473 |
| Aspartate transaminase (U/l) | 21.0 (7.6) | 21.6 (8.4) | 20.3 (6.6) | 0.142 |
| Alanine transaminase (U/l) | 24.5 (13.6) | 25.8 (15.2) | 23.2 (11.7) | 0.098 |
Data are expressed as mean (SD), median (first-third quartile), or number of patients (%). BMI, body mass index. HbA1c, glycated hemoglobin.
The average effective CPAP pressure was 10.3 (2.3) cmH2O. In the 151 women allocated to the CPAP group, the mean nightly use of the device was 4.8 (2.5) hours/night and 113 (75.3%) of them used it for at least 4h/night.
Eighty women (26.1%), 41 in the CPAP and 39 in the control group, had UA levels ≥6mg/dl (average 6.77±0.68mg/dl) at entry, and 227 (73.9%) had values <6mg/dl (average 4.52±0.81mg/dl). Only three women were undergoing allopurinol treatment (one in the CPAP and two in the control group).
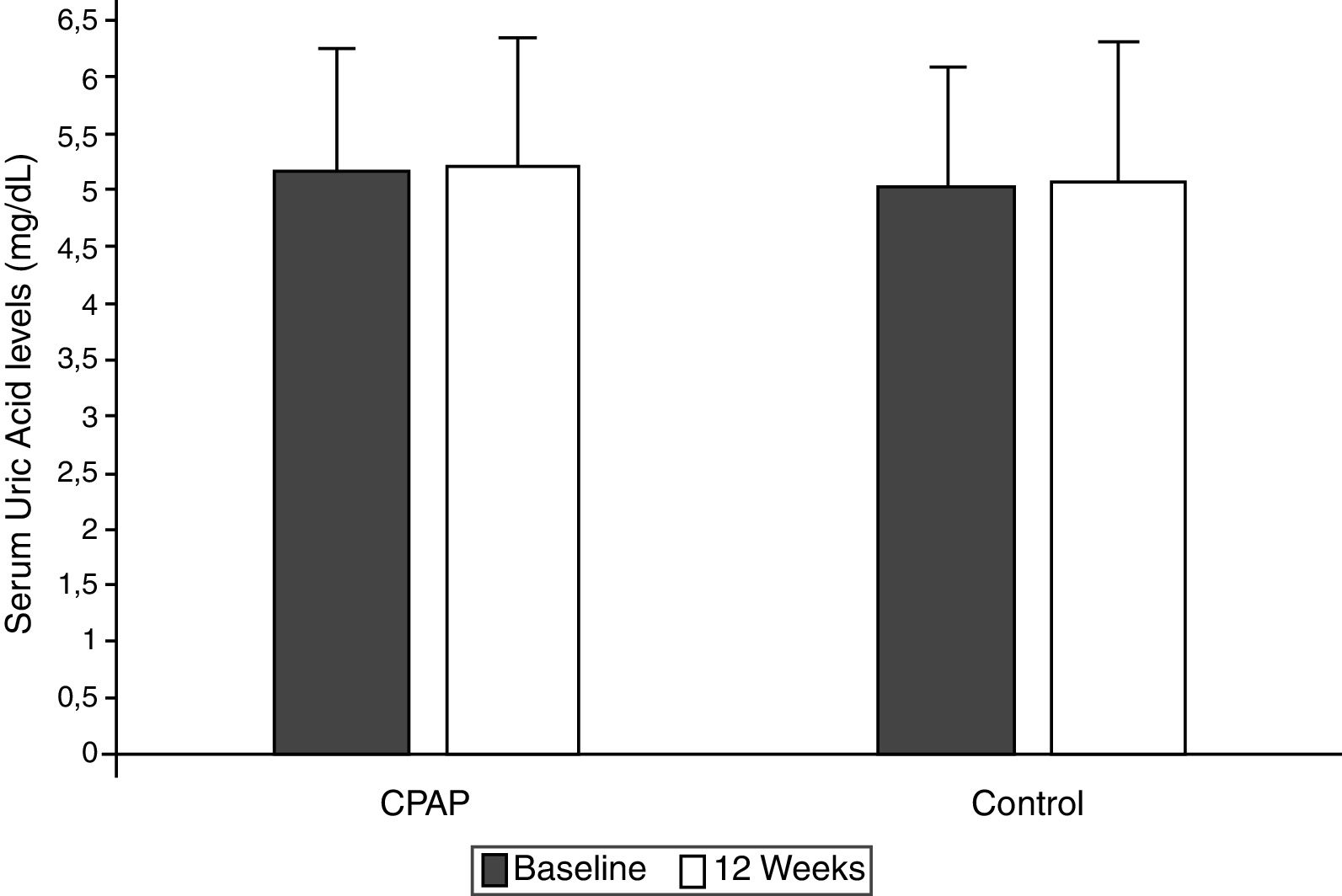
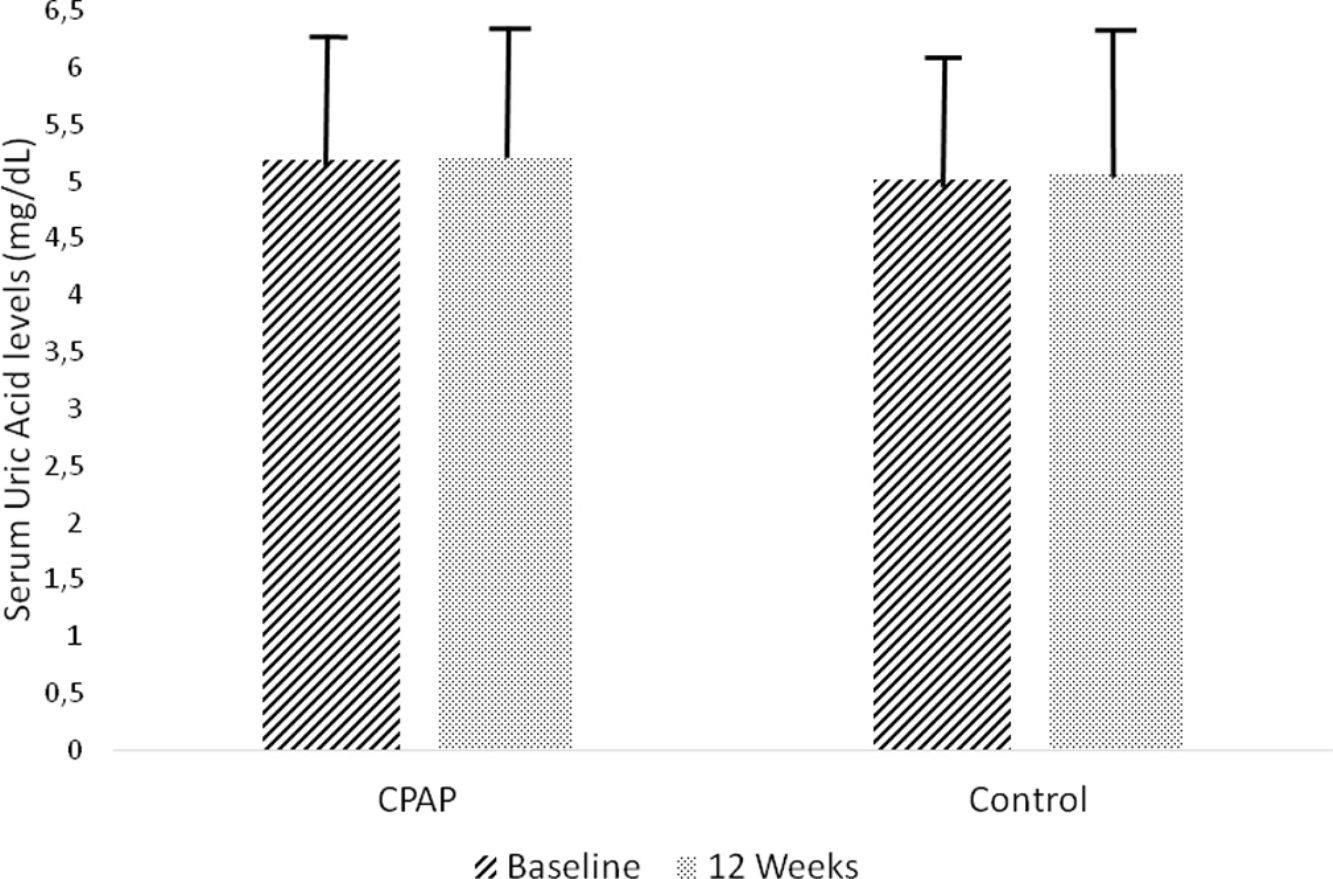
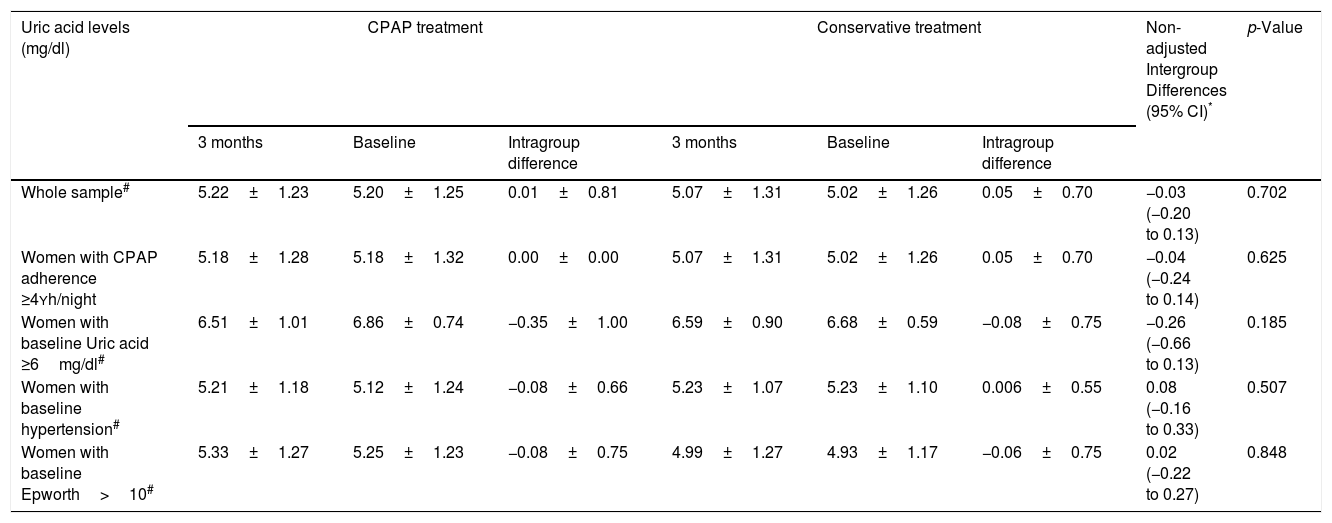
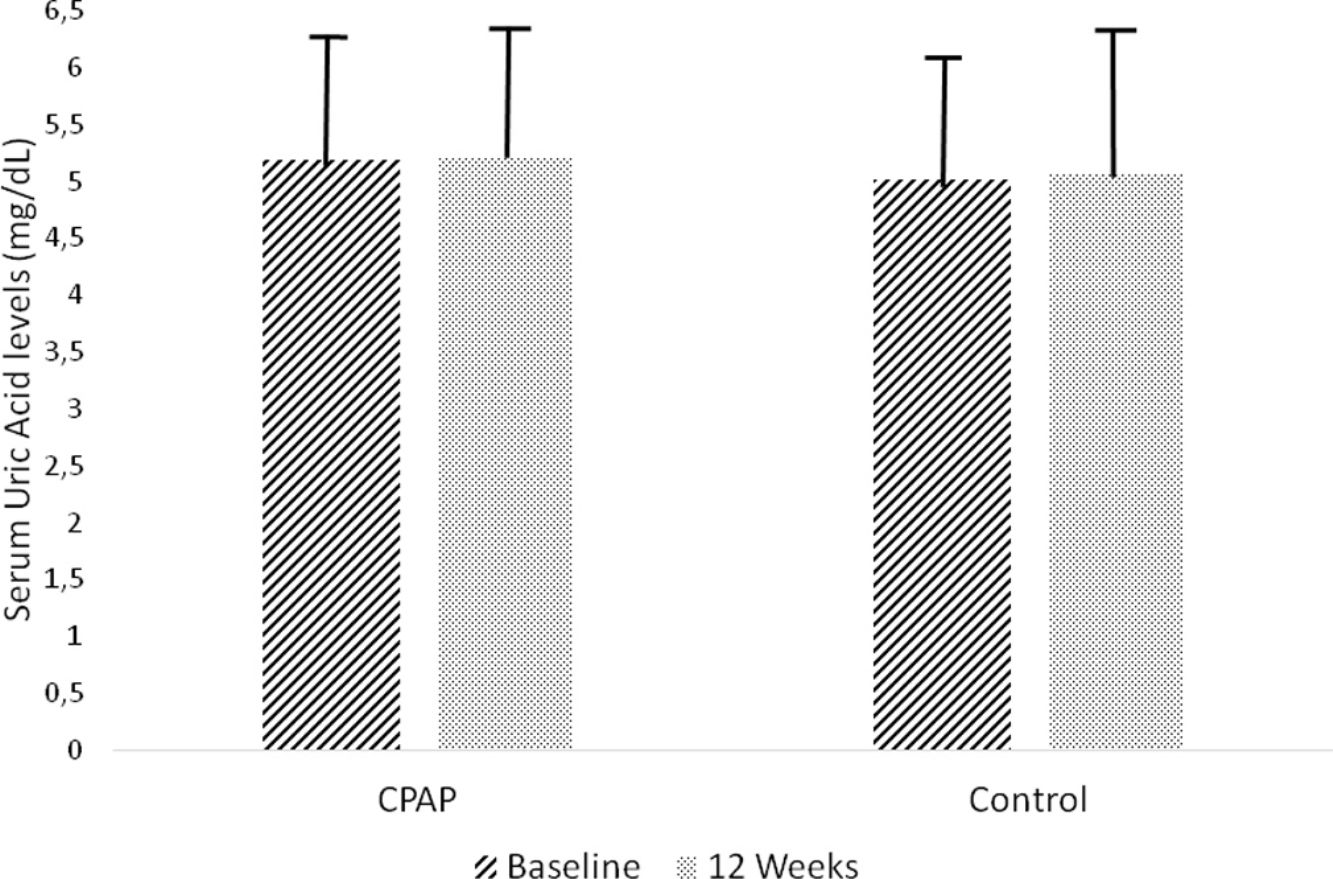
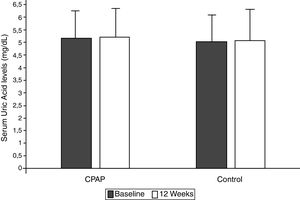
Effect of CPAP therapy on serum UA levelsAfter 12 weeks of follow-up, there was no significant change from baseline UA levels in the CPAP (5.22±1.23 vs. 5.20±1.25mg/dl, intragroup difference 0.01±0.81mg/dl) or conservative treatment group (5.07±1.31 vs. 5.02±1.26mg/dl, intragroup difference 0.05±0.70mg/dl) in the ITT analysis, with non-adjusted intergroup differences of −0.03mg/dl (95%CI −0.20 to 0.13, p=0.702) (Table 2, Fig. 2). These results did not change when they were adjusted for baseline values, age, BMI, and hypouricemiant medication.
Changes in serum uric acid measurements between randomized groups.
| Uric acid levels (mg/dl) | CPAP treatment | Conservative treatment | Non-adjusted Intergroup Differences (95% CI)* | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 3 months | Baseline | Intragroup difference | 3 months | Baseline | Intragroup difference | |||
| Whole sample# | 5.22±1.23 | 5.20±1.25 | 0.01±0.81 | 5.07±1.31 | 5.02±1.26 | 0.05±0.70 | −0.03 (−0.20 to 0.13) | 0.702 |
| Women with CPAP adherence ≥4Yh/night | 5.18±1.28 | 5.18±1.32 | 0.00±0.00 | 5.07±1.31 | 5.02±1.26 | 0.05±0.70 | −0.04 (−0.24 to 0.14) | 0.625 |
| Women with baseline Uric acid ≥6mg/dl# | 6.51±1.01 | 6.86±0.74 | −0.35±1.00 | 6.59±0.90 | 6.68±0.59 | −0.08±0.75 | −0.26 (−0.66 to 0.13) | 0.185 |
| Women with baseline hypertension# | 5.21±1.18 | 5.12±1.24 | −0.08±0.66 | 5.23±1.07 | 5.23±1.10 | 0.006±0.55 | 0.08 (−0.16 to 0.33) | 0.507 |
| Women with baseline Epworth>10# | 5.33±1.27 | 5.25±1.23 | −0.08±0.75 | 4.99±1.27 | 4.93±1.17 | −0.06±0.75 | 0.02 (−0.22 to 0.27) | 0.848 |
The results are expressed as changes in the CPAP versus the control group, at 12 weeks compared to baseline.
Intention-to-treat analysis.
Adjustment for baselines values, body mass index, age, and uric acid lowering medication did not modify these associations: Whole sample, p=0.772; Women with CPAP adherence ≥4h/night, p=0.920; Women with baseline Uric acid levels ≥6, p=0.702; Women with baseline hypertension, p=0.65; Women with Epworth>10, p=0.90
Changes in serum uric acid levels between randomized groups in the intention-to-treat analysis. Non-adjusted treatment effects and 95% confidence intervals (CIs) of continuous positive airway pressure versus conservative treatment at the end of follow-up compared with baseline. Control group (conservative treatment group): Baseline 5.20±1.25mg/dl, 12 weeks 5.22±1.23mg/dl; intragroup difference 0.01±0.81mg/dl. Conservative treatment group: Baseline 5.02±1.26mg/dl, 12weeks 5.07±1.31mg/dl; intragroup difference 0.05±0.70mg/dl. Non-adjusted intergroup difference −0.03mg/dl (95%CI −0.20 to 0.13, p=0.702).
This lack of benefit from CPAP was also observed in the per-protocol analysis, where we included in the CPAP group only those women who used the device for at least 4h/night on average, and in the exclusive analyses of the subgroup of 80 women with baseline hyperuricemia, the subgroup of 96 women with baseline arterial hypertension, and the subgroup of 142 women with excessive daytime somnolence defined as an ESS score>10 (Table 2).
DiscussionThe findings of the present study suggest that 12 weeks of CPAP therapy does not reduce the serum UA levels compared to conservative treatment, in a cohort of unselected women with moderate-to-severe OSA. These results did not change when the analyses were restricted to those women with high baseline UA levels or those with adequate adherence to CPAP.
Oxidative stress, one of the consequences of intermittent hypoxia encountered in OSA patients, is also a common pathway to UA production and it may thus be reflected in UA levels.15 In this respect, hyperuricemia is commonly found in OSA patients, and some indices of OSA severity – namely AHI and minimum SaO2 – have been independently associated with increased levels of UA.6,14,16 A recent study of 1021 individuals found that UA levels were independently associated with OSA, with a 16% increase in the risk of OSA for each 1mg/dL increase in its concentration.3 High serum UA levels are also associated with increased risk of cardiovascular mortality and morbidity, although most of the cardiovascular risk factors potentially overlap with serum uric acid levels concentrations.13 There is some evidence to suggest that hyperuricemia is associated with cardiovascular disease in OSA patients, and that this relationship persists even after controlling for traditional risk factors for cardiovascular disease.6 It can therefore be hypothesized that decreasing UA levels in OSA patients may help to improve the cardiovascular burden associated with OSA.
Although our study did not seek to address the prevalence of hyperuricemia, we found that 26.1% of our sample of women with moderate-to-severe OSA had UA levels ≥6mg/dl, which is lower than the 33% observed in a retrospective cross-sectional Spanish study of 1135 patients evaluated for suspicion of OSA,14 but much higher than the 5.2–7% encountered in two population-based studies.3,17 When we used the cut-off point of 5.7mg/dl used in other studies to define hyperuricemia in women, the prevalence rose to 29.9% (92/307), which was also higher than the 21.6% reported in community-dwelling women by the National Health and Nutrition Examination Survey.13,18
To date, very few studies have investigated the effect of CPAP on this outcome, and most of these had an observational design. Two previous studies have assessed the effect of this therapy on urinary UA excretion and on the urinary UA/creatinine ratio as a marker of nocturnal hypoxia in OSA patients, and they reported that these measures had increased in OSA patients but were normalized after CPAP treatment.2,19 Both studies, however, had an observational design and analyzed small sample cohorts (18 and 30 participants, respectively). Steiropoulos et al. studied 52 males with newly-diagnosed OSA who began CPAP therapy, and they observed that after 6 months of follow-up the group of 32 patients with CPAP use of at least 4h/night significantly reduced their UA levels (8.79±1.48 vs. 6.2±1.37mg/dl, p<0.001), along with other inflammation markers such as total lymphocytes, CD4+ count and tumor necrosis factor α measures, whereas non-compliant patients (<4h/night) did not experience any benefit in the levels of UA (6.62±1.46 vs. 6.71±1.53mg/dl, p=0.223) or other inflammation markers.7 Seetho et al. followed 58 obese OSA patients (28 on CPAP and 30 not on CPAP) for 14 months and observed a reduction in UA levels only in those treated with CPAP, although no correlation was found between change in urate and hours of CPAP use.20 The only RCT in this respect was conducted by Prudon et al. in 38 males with type 2 diabetes mellitus and newly diagnosed OSA, who were randomized to either CPAP or sham-CPAP for 3 months.8 The authors did not find any differences in UA levels between groups at the end of the follow up (p=0.9, 95%CI −28.7 to 29.5).
The present study is the largest RCT undertaken to date to investigate the effect of CPAP therapy on serum UA levels, and the first to focus on women. Our results do not endorse any beneficial effect of 12 weeks of CPAP therapy on UA levels in women with moderate-to-severe OSA, in accordance with the study of Prudon et al. but in contrast to previous observational studies. This lack of benefit cannot be attributable to an overall lack of effect of CPAP, since we have previously demonstrated that this treatment was able to improve several quality of life aspects, including excessive daytime somnolence, and to reduce blood pressure measurements in this same cohort.9,10 Interestingly, the treatment did not even have any benefit in women who had good CPAP adherence with an average use ≥4h/night, or in those with baseline hyperuricemia, suggesting a real lack of effect of OSA treatment on this outcome. Our population had other comorbidities associated with hyperuricemia, and 31.2%, 13.3% and 25.4% of the women fulfilled criteria of hypertension, type 2 diabetes mellitus and hypercholesterolemia at entry, respectively. It seems that OSA treatment itself is insufficient to change the serum urate in patients with several hyperuricemic risk factors. In fact, we have previously reported that 12 weeks of CPAP therapy was not able to improve the glucose and lipid profile, so the lack of effect on UA levels may concur with these findings.10
Our study has limitations. First, we have analyzed a non-selected sample of women in whom the enrolment criterion was moderate-to-severe OSA, regardless of comorbidities. As a result, 73.9% had normal baseline UA levels, so, although our sample size had enough power to demonstrate reductions of at least 0.5mg/dl, there was less likelihood of achieving significant improvements in patients with baseline values within the normal range, due to a possible floor effect. Nevertheless, these results did not change when the 80 women with hyperuricemia were analyzed, although this subgroup may have suffered from the loss of power associated with the smaller sample size (the statistical power decreased to only 39%). In fact, to obtain a statistically significant difference in this group of women with hyperuricemia, a sample size of at least 222 women would have been needed. Furthermore, we cannot rule out that in some women, hyperuricemia was not associated with OSA and, therefore, CPAP therapy may have little effect on this measure. Second, the follow-up period of three months may have been insufficient to achieve improvements in UA values. However, a longer follow-up could have provoked ethical issues, since active treatment was withheld from patients in the control group. Third, the multicenter design may have added some heterogeneity in UA measurement, although, all the centers used the standard uricase method to determine UA levels. Fourth, since the primary endpoint of this trial was quality of life, participants were not given strict dietary counseling with regards to UA, so we cannot rule out the influence of dietary changes on some patients during the follow-up, although we believe that any effect of diet would have been balanced in the two groups, given the RCT design. Fifth, since this cohort comprises only women, it may not be possible to extrapolate the findings to OSA male patients.
In conclusion, we have not found any effect of 12 weeks of CPAP therapy on serum UA levels in an unselected cohort of women with moderate-to-severe OSA, not even in the subgroups with adequate compliance to CPAP or high UA levels at entry. These results do not therefore support the treatment of OSA based on an expectancy of reduction of UA levels.
FundingThis study was supported by a grant from the Spanish Ministry of Economy and Competitiveness, cofounded by Instituto de Salud Carlos III and FEDER funds (PI13/00743), and a grant from the Sociedad Española de Neumología y Cirugía Torácica SEPAR (039/2013). None of these institutions played any role in the objectives, methods, data analysis, or conclusions of the study.
Authorship- -
Conception and design: F.C-R, MA.M-G
- -
Acquisition and interpretation of data: F.C-R, N. R-N, M. G-M, A. S-A, B. J-G, MF. T, A. A-F, J. T-S, J. C-R, M. M-R, A. E-M, MA.M-G, L. S-B, J. N-E, M. S-G, JF. M, MA. S-Q, B. J-C, B. O-B.
- -
Statistical analysis of the data: J. C-G.
- -
Drafting of manuscript and critical revision of major intellectual content: All authors.
- -
Final approval of the version to be published: All authors
The authors declare no conflict of interests.
The Spanish Sleep Network is composed of the following individuals: Juan Santos-Morano (Respiratory Department. Hospital Universitario de Valme. Sevilla. Spain), Monica Gonzalez-Martinez (Respiratory Department. Hospital Universitario Marques de Valdecilla. Santander. Spain), Carmen Carmona-Bernal (Medical-Surgical Unit of Respiratory Diseases, Virgen del Rocio University Hospital. Sevilla. Spain), Nuria Feu-Collado (Department of Respiratory Medicine, Reina Sofia University Hospital. Cordoba, Spain), Paula Rodriguez-Rodriguez (Respiratory Department. Fundación Jimenez Diaz. Madrid. Spain), Irene Cano-Pumarega (Respiratory Department. Hospital Universitario de Getafe. Madrid. Spain), Maria L. Alonso-Alvarez (Respiratory Department. Hospital Universitario de Burgos. Burgos. Spain), Beatriz Galvez-Martinez (Respiratory Department. Hospital Morales Meseguer. Murcia. Spain), Nuria Reina-Marfil (Respiratory Department. Hospital Universitario Virgen de la Victoria. Malaga. Spain), Maria J. Selma (Respiratory Department. Hospital Universitario y Politecnico La Fe. Valencia, Spain), Lucia L. Quintana-Hidalgo (Laboratory Department. Hospital Dr. Negrin. Gran Canaria. Spain), Antonia Llunell-Casanovas (Respiratory Department. Consorcio Sanitario de Terrassa. Barcelona. Spain), Jaime Corral (Respiratory Department. Hospital San Pedro de Alcantara. Caceres. Spain), Andrea Crespo Sedano (Respiratory Department. Hospital Rio Hortega, Valladolid. Spain).