Primary ciliary dyskinesia (PCD) is characterized by an alteration in the ciliary structure causing problems in the clearance of respiratory secretions.1,2 It is an autosomal recessive hereditary disease, and up to 40 causative genes have been described in >70% of patients.3 It is difficult to confirm a diagnosis of PCD using currently available techniques, and the European guidelines recommend a combination of tests.4 The detection of low levels of nasal nitric oxide (nNO) is a useful screening tool,4,5 but this method is only validated in patients older than 5 years of age, and may be normal in some cases.4,5 Ciliary ultrastructure analysis with electron microscopy gives false positives, related to secondary changes caused by respiratory infections, as well as false negatives, and may be normal in 21% of cases.4 The analysis of ciliary beat pattern using high-speed video-microscopy is very useful for diagnosis.4,6 However, it also gives false positives due to respiratory infections, there is a lack of standardization in the preparation of the samples, it has to be interpreted by experienced personnel, and it has an element of subjectivity.4
In addition to molecular diagnostics,3 immunofluorescence has been identified as a technique that can help define the specific PCD defect and improve diagnosis.7 The aim of this article is to report the cases of 2 sisters that show the usefulness of combining these techniques to reach an accurate diagnosis of the protein and molecular defect causing PCD.
The study was approved by the Ethics Committee, and authorization for inclusion in the study was requested from the parents and the patients.
Case 1. A 16-year-old girl, born at term. She was admitted at 3 days of life for bronchiolitis, and subsequently developed recurrent otitis media, chronic rhinitis, recurrent bronchitis, and middle lobe bronchiectasis. At 4 years of age, an electron microscopy study showed a loss of 40% of the outer dynein arm (ODA) and 70% of the inner dynein arm (IDA), confirming a diagnosis of PCD. Haemophilus influenzae and Pseudomonas aeruginosa were habitually isolated from sputum cultures, so the patient was treated with nebulized colistin, respiratory physiotherapy, and the administration of 7% nebulized hypertonic saline. Spirometry (GLI-2012 reference values)8,9 showed FVC 3.36l (z-score −0.08), FEV1 2.23l (z-score −2.21), FEV1/FVC 66% (z-score −2.83), FEF25%–75% 1.42l/s (z-score −3.19).
Case 2. A 13-year-old girl, born at term. She was admitted at 11 days of life for bronchiolitis, and subsequently developed pneumonia in the right upper lobe and recurrent bronchitis. At 12 months of life, electron microscopy showed a 30% loss of ODA and a 70% loss of IDA, so she too was diagnosed with PCD. Chest computed tomography scan revealed atelectasis in the middle lobe and lingula, peribronchial thickening and heterogeneous aeration. Sputum cultures were positive for H. influenzae. She was treated with respiratory physiotherapy and 7% nebulized hypertonic saline. Spirometry showed FVC 1.97l (z-score −0.91), FEV1 1.45l (z-score −2.18), FEV1/FVC 73% (z-score −2.18), FEF25%–75% 0.98l/s (z-score −3.12).
Last year, our patients were re-evaluated using newly available diagnostic techniques. The nNO value was very low in both girls: 53.3ppb (15.2VNOnl/min) and 61ppb (17.2VNOnl/min). Samples of ciliated respiratory epithelium were collected from the lower nasal meatus with a 2mm brush for video-microscopy and immunofluorescence studies, and samples of peripheral blood were obtained for genetic studies.
We analyzed ciliary beat frequency and pattern with high-speed video (MotionPro® X4, IDT, CA, USA) coupled to an optical microscope: absence of ciliary motility could be seen in both sisters.
For the genetic study, genomic DNA was extracted from the peripheral blood of both patients and their parents. The samples of 1 of the sisters and the parents were analyzed with TruSight One Sequencing Panel (Illumina, San Diego, CA, USA) and sequenced with the MiSeq platform (Illumina). This panel included 20 genes associated with PCD. The results were analyzed using the VariantStudio v2.2.1, Alamut Visual v2.11, and VarSome programs and different predictors of pathogenicity. We consulted allele frequency in the Genome Aggregation Database and scientific evidence of pathogenicity in the Human Gene Mutation Database. The candidate variants were confirmed for both this patient and her sister using Sanger sequencing. The sisters show a compound heterozygous mutation in the DNAH5 gene for variants not previously described, but probably pathogenic: c.4625_4628delGAGA:p.(Arg1542ThrfsTer6) and c.12706-2A>T. Parents are heterozygous for 1 of the mutations. This gene encodes one of the ODA heavy chains and is essential to ciliary function.10
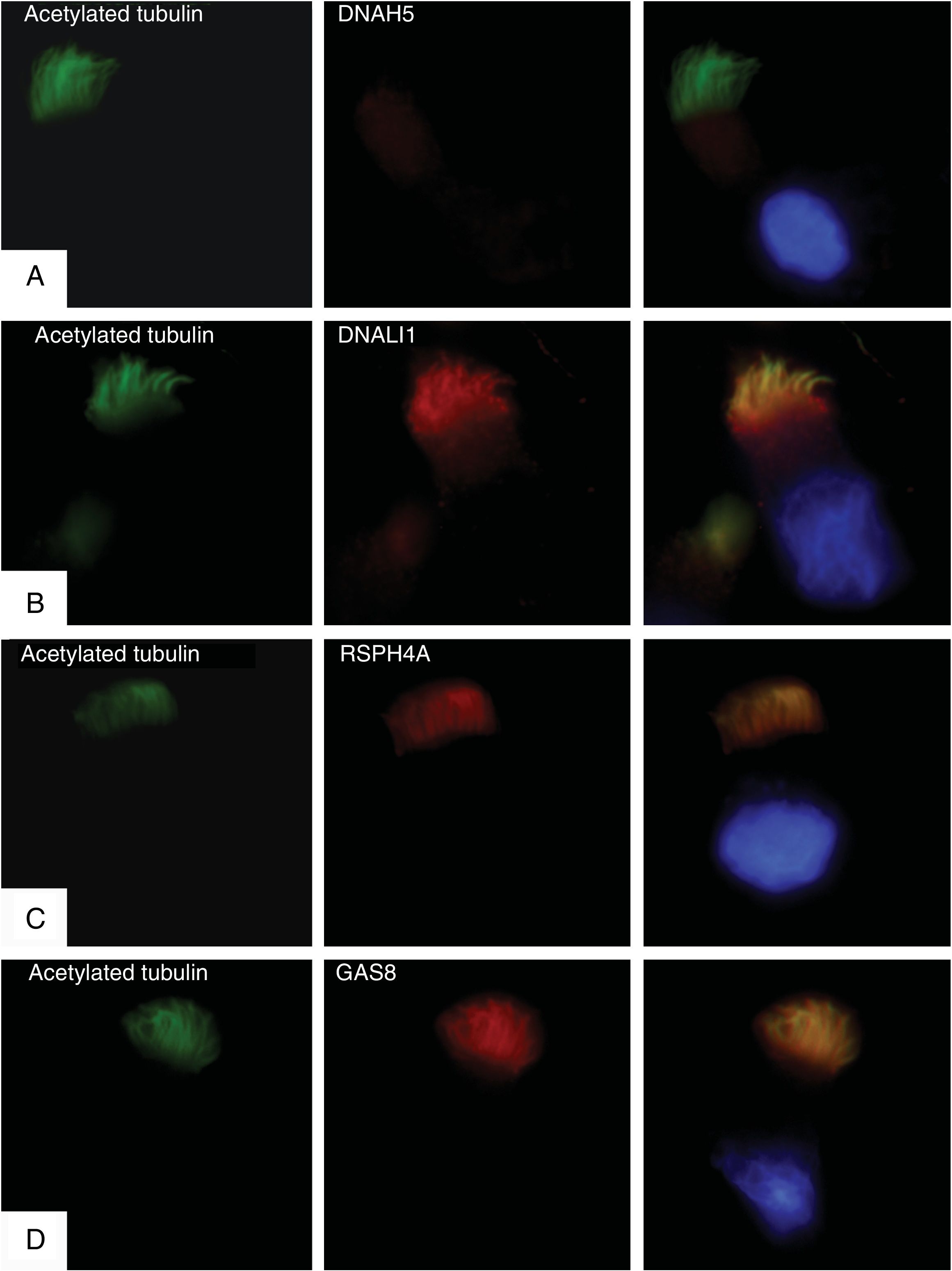
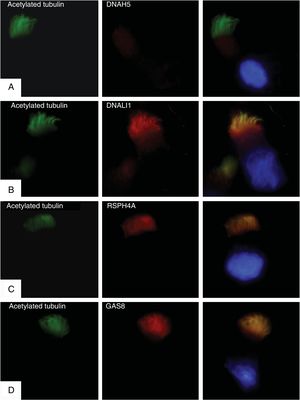
Immunofluorescence studies were conducted in respiratory epithelial cells to confirm expression or absence of the mutated DNAH5 protein. Primary cilia anti-acetylated tubulin antibodies (Sigma Aldrich, St. Louis, MO, USA) and 4 ciliary structure proteins were used: DNAH5 (ODA); DNALI1 (IDA); RSPH4A (radial connections), and GAS8 (nexin-dynein-regulatory complex). The results showed a complete absence of DNAH5 protein in the ciliary axoneme (Fig. 1A) and colocalization of DNALI1, RSPH4A and GAS8 with the ciliary acetylated tubulin (Fig. 1B–D).
Immunofluorescence analysis of the ciliary ultrastructure. The first column shows the presence of cilia in the cell using acetylated tubulin (in green), the second shows the outcome of incubation with primary ciliary protein antibodies (in red), and the third shows the merge of tubulin with each ciliary protein and the DAPI-stained nucleus (blue). (A) Absence of protein DNAH5 (external component of dynein arms) in the ciliary axoneme. (B–D) Presence and colocalization of tubulin and ciliary axoneme proteins (in yellow): (B) DNALI1 (external component of dynein arms); (C) RSPH4A (radial spoke head component), and (D) GAS8 (nexin-dynein regulatory complex component).
The results of the immunofluorescence test are consistent with the genetic study and confirm that the previously described mutations produce a complete lack of expression of the protein DNAH5 in the ciliary axoneme and cause an ODA defect.11,12 In our analysis of video-microscopy, ciliary immobility was observed, which is consistent with the findings in these cases.11 The observation on electron microscopy of an alteration in IDA as well as ODA could be explained by IDA changes due to respiratory infections,13 the fact that in healthy subjects IDA might not occur in more than 50% of the doublets,14 or the presence of processing artifacts.15
The immunofluorescence test has limitations4,7: it does not provide antibodies to all defective proteins, and the technique may fail due to the absence of cilia or interference of mucus or blood in the sample. However, in combination with the molecular study, it offers a useful approach to the diagnosis of this disease and to identifying the specific causative defect. This conclusion will have to be confirmed in more extensive studies.
FundingThis work has been partially funded by a Strategic Action in Health grant from the Instituto de Salud Carlos III(PI16/01233), a grant from the Spanish Society of Pediatric Pulmonology, and a grant from the Catalan Foundation of Pulmonology (FUCAP).
Conflict of interestsAMG has received funding for participation in Abbvie advisory boards, and has received assistance for travel and registration at medical congresses from Abbvie, Actelion, and Novartis, all activities unrelated with this work. SR has received assistance for travel and registration at medical congresses from Abbvie, Teva, and Novartis, all activities unrelated with this work. The other authors state that they have no conflict of interests.
The authors are involved in the Action COST BM1407 Translational research in primary ciliary dyskinesia: bench, bedside, and population perspectives (BEAT PCD). This work has been carried out in the framework of the doctoral program in Pediatrics, Obstetrics and Gynecology at the Universitat Autònoma de Barcelona.
Please cite this article as: Baz-Redón N, Rovira-Amigo S, Camats-Tarruella N, Fernández-Cancio M, Garrido-Pontnou M, Antolín M, et al. Papel de la inmunofluorescencia y el diagnóstico molecular en la caracterización de la discinesia ciliar primaria. Arch Bronconeumol. 2019;55:439–441.