Red cell distribution width (RDW) describes heterogeneity in the size of red blood cells. An increase in RDW has been associated with excess mortality in heart failure and other chronic diseases. Since there is an increased risk of cardiovascular morbidity and mortality in obstructive sleep apnea (OSA), it is possible that these patients have a high RDW.
MethodWe recruited subjects aged 18–60 years referred to the sleep-disordered breathing unit for suspected OSA. Subjects with any comorbidity were excluded. Apnea-hypopnea index (AHI) was calculated from the respiratory polygraphy. The RDW was obtained from the complete blood count. Changes in RDW after one year of treatment with continuous positive airway pressure (CPAP) were determined.
ResultsWe included 34 healthy subjects and 138 with OSA, aged 40.5±9.8 and 45.6±9.2 (P=.004) years, respectively. The RDW was higher in subjects with OSA compared to healthy subjects: 13.40 (12.40–14.40) vs. 13.15 (12.07–14.23) (P=.036). AHI showed a positive independent relationship with RDW in both the whole population (r=0.223; P=.002) and the OSA group (r=0.231; P=.005). No significant changes were found in RDW after one year of CPAP therapy.
ConclusionsRDW increase in patients with OSA is directly associated with severity, although levels are not modified by the effective treatment of OSA with CPAP.
El ancho de distribución eritrocitaria (ADE) describe el grado de heterogeneidad en el tamaño de los hematíes. Un incremento de ADE se ha asociado con exceso de mortalidad en insuficiencia cardiaca y otras enfermedades crónicas. Dado que existe mayor riesgo de morbimortalidad cardiovascular en apnea obstructiva del sueño (AOS), es posible que estos pacientes presenten un ADE elevado.
MétodoSe reclutaron sujetos de 18 a 60 años remitidos a la Unidad de Trastornos Respiratorios del Sueño (UTRS) por sospecha de AOS. Se excluyeron sujetos con cualquier comorbilidad. En la poligrafía respiratoria se determinó el índice de apnea-hipopnea (IAH). El ADE se obtuvo a partir del hemograma. Al año de seguimiento se determinaron los cambios de ADE tras tratamiento con presión positiva continua en la vía respiratoria (CPAP).
ResultadosSe incluyeron 34 sujetos sin AOS y 138 con AOS con una edad de 40,5±9,8 y 45,6±9,2 (p=0,004) respectivamente. El ADE fue mayor en sujetos con AOS que en sujetos sanos: 13,40 (12,40-14,40) vs 13,15 (12,07-14,23) (p=0,036). El IAH mostró una relación positiva e independiente con ADE tanto en el conjunto de la población (r=0,223; p=0,002) como en el grupo con AOS (r=0,231; p=0,005). No se observaron cambios significativos de ADE tras un año de tratamiento con CPAP.
ConclusionesEl ADE está aumentado en AOS en relación directa con su gravedad, sin embargo, sus niveles no se ven modificados por el tratamiento efectivo de la AOS con CPAP.
Obstructive sleep apnea (OSA) is a very common condition in the general population, and is associated with loss of quality of life, arterial hypertension, cardiovascular and cerebrovascular diseases, traffic accidents, and increased mortality.1,2 In Spain, between 3% and 6% of the population have symptomatic sleep apnea-hypopnea syndrome (SAHS) and 24%–26% have OSA, defined as more than 5 nocturnal apnea-hypopnea events per hour (AHI>5).3 OSA is associated with variable degrees of hypoxemia, hypercapnia, reduced intrathoracic pressure, and sympathetic and cortical central nervous system activation. These intermediary mechanisms can potentially predispose to the development of cardiovascular and metabolic diseases and premature death. Currently, the diagnosis of clinically suspected OSA must be confirmed with sleep studies. To date, no clinically useful variables for cardiovascular risk specifically associated with OSA have been identified, but such biomarkers would be of great benefit in assisting the physician's decision-making process; for example, when to perform a sleep study or whether to start treatment or monitor the patient instead. Spanish guidelines on the management of OSA recommend a metabolic profile, including complete blood count, basic biochemistry parameters and a lipid profile in all patients undergoing a sleep study.1
One of the variables routinely analyzed in a complete blood count is red cell distribution width (RDW). RDW is a quantitative measure of the variability of red cell sizes. It is obtained using a mathematical calculation (Fig. 1), so the cost is negligible. Its main application at present is for the differential diagnosis of anemia,4 and values are not affected by the patient's nutritional status, sex or age.5 Recent studies show that increased RDW, even with normal reference ranges, is strongly associated with a greater risk of death and cardiovascular disease in middle-aged and elderly individuals.6–15 RDW can act as a biomarker for cardiovascular risk that is sensitive to systemic inflammatory changes and to mobilization of iron deposits during oxidative stress.11 A relationship between RDW and OSA severity has been described in patients with and without associated cardiovascular disease,16–18 and RDW has been reported to diminish with the use of continuous positive airway pressure (CPAP).19 However, these studies include limited numbers of patients, follow-up is short, and most include patients with comorbidities other than OSA.
We postulated that RDW may be a biomarker that can identify the presence and severity of OSA in patients with suspected sleep-disordered breathing.
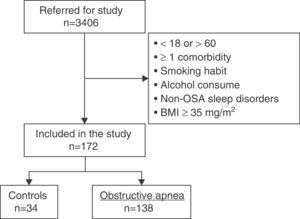
MethodsStudy SubjectsData from a prospective study on epigenetic abnormalities in OSA currently under analysis (EPIOSA, ClinicalTrials.gov: NCT02131610) were used. The study included patients aged 18–60 years referred to the Sleep-Disordered Breathing Unit (SDBU) of the Hospital Universitario Miguel Servet, Zaragoza with suspected OSA between February 2013 and July 2014. The general methodology and inclusion/exclusion criteria of this study have been described elsewhere.20 In summary, the following subjects were excluded: active smokers or individuals with a smoking history of >5 pack-years, regular consumption of alcohol (mean ≥20g/day), and subjects with sleep disorders other than OSA or any comorbidity requiring the regular administration of any drug. Patients with blood pressure ≥140/90mmHg, blood glucose >126mg/ml or body mass index (BMI) ≥35kg/m2 in the first visit were also excluded. The study protocol was approved by the Ethics and Clinical Research Committee of Aragon (03/2013).
ProceduresIn the first visit, all subjects underwent a standardized protocol of study tests as follows: (1) clinical questionnaires (general health, nutrition, quality of life, daytime sleepiness, comorbidities, sleep hygiene); (2) anthropometrics and general examination (blood pressure, weight, height, and neck, waist and hip circumferences); (3) cardiorespiratory polygraph at home; (4) spirometry; (5) ultrasonography of internal carotid arteries, carotid sinus, and common carotid artery; (6) full blood analysis. RDW was determined using a blood cell counter (Coulter® LH 780). Additional tests, including complete blood count, were repeated in all patients after 1 year, irrespective of OSA diagnosis, severity or treatment received. Polygraphy was performed and analyzed according to SEPAR recommendations and the Spanish SAOS1 initiative.1
The diagnosis of OSA and severity were established according to the number of apneas and hypopneas per hour of recording (apnea/hypopnea index [AHI]). Subjects with an AHI<5 were considered healthy. After receiving a diagnosis of OSA, patients were prescribed CPAP according to the routine recommendations.1 A patient was considered appropriately treated when CPAP was delivered for an average of more than 4h/day throughout the follow-up period. CPAP use was evaluated in each visit by recording the readings on the CPAP unit counter.
Statistical AnalysisCategorical variables are shown as case numbers and percentages. The Kolmogorov–Smirnov test was used to assess the distribution of the quantitative variables. Variables with normal distribution are expressed as mean±standard deviation and variables with non-normal distribution as median and interquartile range. Comparison between groups was performed using the Student t test and ANOVA or the Mann–Whitney U test and Kruskal–Wallis test, depending on the distribution of the variable. Differences between variables with categorical data were established using the Chi-squared test. The relationship between the individual RDW value and other variables of clinical interest was evaluated using adjusted linear regression models. Statistical analyses were performed using version 20 of the SPSS statistical package (IBM Corporation, Somers NY, USA).
ResultsCharacteristics of Study PopulationFig. 2 shows the study patient disposition. A total of 172 individuals meeting the inclusion/exclusion criteria were included in the final analysis. Table 1 describes the population characteristics, distributed by presence or absence of OSA.
Baseline Characteristics of Study Subjects.
| No OSA (n=34) | OSA (n=138) | P | |
|---|---|---|---|
| Age, years | 40.5±9.8 | 45.6±9.2 | .004 |
| Men, n (%) | 19 (52.8%) | 110 (80.9%) | .001 |
| BMI, kg/m2 | 25.9±3.3 | 31.1±4.1 | .000 |
| Epworth | 10.3±5.0 | 10.3±4.8 | .973 |
| SBP, mmHg | 118.5±15.3 | 129.3±14.9 | .000 |
| DBP, mmHg | 74.0±11.8 | 80.1±11.4 | .005 |
| FVC, l | 4.48±0.91 | 4.38±1.06 | .611 |
| FEV1, l | 3.59±0.82 | 3.46±0.8 | .369 |
| Total cholesterol, mg/ml | 200±36.3 | 214.2±39.1 | .05 |
| LDL cholesterol, mg/ml | 120 (72–162) | 132 (89–175) | .09 |
| HDL cholesterol, mg/ml | 54.2±9.3 | 48.5±10.5 | .004 |
| Triglycerides, mg/dl | 93.5 (50.5–136.5) | 128.5 (42.5–214.5) | .000 |
| Blood glucose, mg/dl | 86.5 (76.5–96.5) | 92 (73–111) | .017 |
| Creatinine, mg/dl | 0.82±0.15 | 0.87±0.14 | .106 |
| Hb, g/l | 14.6 (12.7–16.5) | 14.9 (13.6–16.2) | .058 |
| CRP, mg/dl | 0.08 (0–0.24) | 0.23 (0–0.58) | .000 |
| RDW, % | 13.15 (12.07–14.23) | 13.40 (12.40–14.40) | .036 |
Variables with normal distribution are expressed as mean±standard deviation and variables with non-normal distribution are expressed as median (interquartile range).
BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSA, sleep obstructive apnea; PYI, pack-year index; RDW, red cell distribution width; SBP, systolic blood pressure.
Ninety-two percent of the subjects were of Caucasian ethnic origin. Controls with OSA were on average older (40.5 vs. 45.6 years P=.004), with higher BMI (26 vs. 31, P<.001) and significantly higher levels of blood pressure, cholesterol, triglycerides, blood glucose, C-reactive protein (CRP) (P<.05 for all differences).
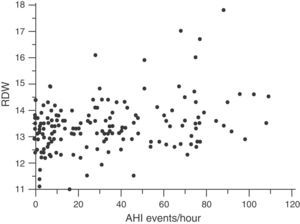
Red Cell Distribution WidthMedian RDW and interquartile range in the overall population at the beginning of the study were 13.3 (12.3–14.3): in healthy subjects, 13.15 (12.07–14.23) and in OSA patients, 13.40 (12.40–14.40) (P=.036). The correlation between RDW and baseline characteristics of the study subjects is shown in Table 2. RDW is significantly associated with the following: BMI (r=0.190; P=.014); CRP (r=0.197; P=.011); time SaO2<90% (CT90) (r=0.215; P=.007); and AHI (r=0.212; P=.006) (Fig. 3). Table 2 also shows an adjusted multiple regression model in which the presence of age, hemoglobin (Hb) and BMI are forced. RDW maintained a positive independent relationship with AHI (r=0.223; P=.002) and with CT90 (r=0.232; P=.002). In these models, BMI and CRP also persist as independent variables associated with RDW. A secondary analysis was performed among OSA patients only to establish relationships between RDW and OSA severity. After adjusting for the multiple regression model, RDW remains significantly associated with CT90 (r=0.255; P=.002) and AHI (r=0.231; P=.005). In this analysis of OSA patients only, BMI and CRP are not independently related with RDW.
Relationship of RDW With Baseline Variables in Overall Population (n=172).
| Linear Regression | Multiple Regression (Model With AHI) | Multiple Regression (Model With CT90) | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Age, years | 0.082 | .291 | ||||
| BMI, kg/m2 | 0.190 | .014 | 0.181 | .01 | 0.151 | .03 |
| Epworth | 0.151 | .054 | ||||
| PYI | 0.071 | .366 | ||||
| SBP, mmHg | 0.022 | .783 | ||||
| DBP, mmHg | 0.085 | .280 | ||||
| Total cholesterol, mg/ml | −0.014 | .857 | ||||
| LDL cholesterol, mg/ml | 0.095 | .232 | ||||
| HDL cholesterol, mg/ml | −0.150 | .053 | ||||
| Triglycerides, mg/dl | −0.005 | .949 | ||||
| Blood glucose, mg/dl | 0.109 | .163 | ||||
| Creatinine, mg/dl | −0.038 | .627 | ||||
| Hb, g/l | −0.074 | .343 | ||||
| CRP, mg/dl | 0.197 | .011 | 0.236 | .001 | 0.146 | .035 |
| CT90 | 0.215 | .007 | – | – | 0.232 | .002 |
| AHI | 0.212 | .006 | 0.223 | .002 | – | – |
AHI, apnea-hypopnea index; BMI, body mass index; CRP, C-reactive protein; CT90, time SaO2<90%; DBP, diastolic blood pressure; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PYI, pack-year index; RDW, red cell distribution width; SBP, systolic blood pressure.
Table 3 shows the characteristics of the participants at the initial visit and after 1 year of follow-up. Of the 34 healthy subjects, 32 attended for re-evaluation.
Baseline Characteristics and After 1 Year of Follow-Up in Healthy Subjects and Patients Treated or Not Treated with CPAP.
| No OSA (n=32) | OSA Not Treated With CPAP (n=44) | OSA Treated With CPAP (n=71) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Year | P | Baseline | 1 Year | P | Baseline | 1 Yeara | P | |
| AHI | 2.1±1.3 | 2.3±2.1 | .648 | 28±45 | 29±43 | .144 | 44±39 | 4.7±3.9 | <.001 |
| Epworth | 10±5.5 | 8.3±4.6 | .029 | 9.1±4.3 | 7.1±4.1 | .003 | 11.5±4.8 | 6.9±5.1 | .000 |
| BMI, kg/m2 | 25.5±3.1 | 25.6±3.3 | .749 | 28.4±4.4 | 28.5±4.5 | .687 | 33.1±5.1 | 32.7±4.8 | .025 |
| SBP, mmHg | 117.9±16.5 | 112.4±13.9 | .055 | 122.7±14.1 | 121.9±11.8 | .701 | 132.7±13.7 | 131.4±14.1 | .468 |
| DBP, mmHg | 73.7±11.7 | 68.5±9 | .007 | 74.1±10.3 | 73.7±8.7 | .815 | 83.4±11.03 | 78.1±9.9 | .001 |
| Total cholesterol, mg/ml | 201.5±39 | 201.3±39.1 | .977 | 211.6±37.9 | 210±32.4 | .648 | 216.7±39.8 | 211.9±36.1 | .248 |
| LDL cholesterol, mg/ml | 119 (102–141) | 124 (98–141) | .864 | 129 (109–156) | 128 (113–146) | .971 | 137 (117–161.5) | 129 (110–152) | .205 |
| HDL cholesterol, mg/ml | 54.3±9.1 | 52.9±10.5 | .303 | 51.1±9.6 | 50.8±10.9 | .790 | 46.8±11.2 | 46.9±10.4 | .830 |
| Triglycerides, mg/dl | 94 (76–122) | 92 (71–125) | .546 | 114 (88–167) | 125 (81–168) | .683 | 137 (102–192) | 140 (98–194) | .425 |
| Blood glucose, mg/dl | 88 (82–97) | 84 (76–93) | .039 | 89 (82–101) | 90 (85–98) | .054 | 95 (85–102) | 95 (90–102) | .529 |
| Creatinine, mg/dl | 0.83±0.12 | 0.83±0.13 | .823 | 0.86±0.14 | 0.83±0.12 | .098 | 0.8±0.14 | 0.8±0.14 | .979 |
| Hb, g/l | 16.6 (13.4–15.2) | 14.3 (13.5–15.1) | .534 | 15 (14.3–15.6) | 15.2 (14.3–15.5) | .447 | 14.9 (14.3–15.7) | 14.7 (13.8–15.4) | .042 |
| CRP, mg/dl | 0.09 (0.04–0.23) | 0.11 (0.05–0.27) | .673 | 0.16 (0.1–0.35) | 0.18 (0.09–0.52) | .006 | 0.28 (0.14–0.49) | 0.38 (0.15–0.57) | .540 |
| RDW, % | 13.1 (12.4–13.6) | 13.2 (12.6–13.5) | .942 | 13.3 (12.8–13.7) | 13.3 (12.9–13.7) | .571 | 13.4 (13.05–14.1) | 13.6 (13.1–14.05) | .234 |
Variables with normal distribution are expressed as mean±standard deviation and variables with non-normal distribution are expressed as median (interquartile range).
AHI, apnea-hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; CRP, C-reactive protein; DBP, diastolic blood pressure; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; Hb, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSA, sleep obstructive apnea; PYI, pack-year index; RDW, red cell distribution width; SBP, systolic blood pressure.
None of the OSA patients were lost-to-follow-up. Of these, 85 began CPAP treatment. Seventy-one of these adhered appropriately to treatment and were included in the group “OSA treated with CPAP”.
Of the other 14 patients who began CPAP, 4 subsequently rejected this treatment and the other 10 were withdrawn due to lack of compliance. These 14 patients were included in the group “OSA not treated with CPAP”. The remaining patients in this group did not receive CPAP as they did not meet the medical criteria (n=21), or because they refused to use the system (n=9).
Not included in this table are data from 23 OSA patients who received alternative treatment to CPAP (18 received different forms of upper airway surgery, and 2 used mandibular advancement devices) and 3 who refused a repeat CRP determination. After 1 year, no changes were observed in study variables, including RDW, in healthy subjects or in OSA patients not treated with CPAP. In OSA patients treated with CPAP, daytime sleepiness measured according to the Epworth questionnaire and diastolic pressure reduced significantly during the 1-year study period, while RDW levels were not significantly affected. A subanalysis grouping patients according to their OSA severity, based on AHI (i.e., 5–15, 15–30, or >30), did not reveal any RDW changes, irrespective of whether they had received CPAP treatment or not.
DiscussionThe main finding of the study suggests that RDW is greater in patients with OSA than in healthy subjects, and that it is independently associated with the level of OSA severity determined by AHI or by recorded time of hypoxemia. However, further studies are required to determine the potential utility of RDW as a biomarker capable of distinguishing between healthy subjects and those with OSA. The other result of interest is that RDW levels are not modified with effective CPAP treatment. RDW has recently been evaluated as a possible biomarker in various types of heart disease, due to its positive relationship with cardiovascular death.7–14 The few studies that have evaluated the relationship between RDW and OSA suggest that RDW levels rise in association with the degree of severity of OSA.16–19 The hypothesis proposed to explain high RDW levels in OSA is the presence of systemic inflammation that mobilizes iron deposits, and, as a result, may lead to increased RDW values. The association between OSA and systemic inflammation continues to be a topic of debate. Research primarily conducted in laboratory animals indicates that intermittent hypoxia plays a role in the development of accelerated atherosclerosis, and one of the main intermediate mechanisms of this is systemic inflammation.21 Carotid ultrasonography has been used to show how CPAP reduces carotid intima-media thickness as an expression of improvement in subclinical atherosclerosis.22 However, controlled studies have not shown any reduction in the level of systemic inflammation evaluated using various proinflammatory cytokines after 6 months of treatment with CPAP.23 Thus, the role of inflammation as a factor explaining the consistent increase in cardiovascular morbidity and mortality in OSA patients is currently in question.2 Data from our study indicate that CRP levels are higher in OSA patients than in healthy individuals; however, effective CPAP treatment did not help reduce CRP or RDW levels. This supports the findings of the randomized trial by Stradling et al.,23 in which CPAP failed to reduce OSA-related systemic inflammation.
The finding of a positive correlation between RDW and CRP in the overall population and among OSA patients reinforces the idea that RDW may be a biomarker for systemic inflammation (Table 2). However, no independent relationship between RDW and CRP was identified in OSA patients. This suggests that these 2 biomarkers, at least in OSA, reflect different physiopathological processes. After 1 year of CPAP treatment, RDW remained unchanged in OSA patients, so our results do not confirm previous results in which RDW was modified with the use of CPAP.19 In this study, follow-up was only 6 months, the population was smaller (n=58), only patients with AHI>30 were included, and most patients had other comorbidities confounding the interpretation of results that RDW, like other circulating biomarkers such as CRP, do not change with CPAP. Our results do not confirm the effect of CPAP on RDW levels. However, the study populations are different, since our patients were selected with the aim of avoiding potential causes of systemic inflammation other than OSA, thus avoiding bias in interpretation. The lack of effect of CPAP on RDW did not indicate a lack of treatment efficacy. On the contrary, after 1 year of therapy, OSA patients receiving CPAP showed statistically significant reductions in Epworth scale values and diastolic blood pressure figures, findings similar to those repeatedly described in association with CPAP.24
Some limitations must be taken into account when interpreting the results of this study, mainly its observational nature and the lack of randomization to evaluate the effect of CPAP. However, the sample size was, according to the literature, sufficient to allow changes due to the CPAP intervention to be detected. The groups of healthy subjects and OSA patients are not fully comparable, since the healthy subjects were somewhat younger, more of them were women, and they presented a lower BMI, lower blood pressure and lower blood lipid levels.
The inclusion/exclusion criteria of our series are also very restrictive, and the population was limited to subjects aged 18–60 years. This prevents any generalization of results to the overall OSA population.
On the other hand, as the screening of participants was restrictive, selection bias was avoided (subjects with chronic diseases associated with an increase in RDW were excluded), so robust associations between RDW and OSA can be established.
ConclusionsRDW was higher among a population of OSA patients with no associated comorbidity than among healthy individuals without OSA. The RDW level was independently associated with the severity of OSA in the absence of associated comorbidity.
However, RDW was not modified by effective treatment of OSA with CPAP, and this study brings into question the clinical utility of RDW in the management of patients with OSA.
FundingEPIOSA is a research project funded by the Instituto de Salud Carlos III (PI12/01275), the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR 71/2012), and the Aragon Respiratory Tract Society (SADAR 02/2013).
AuthorshipLeón E. had access to the data, completed them and entered them in a database, and subsequently performed the statistical analysis. León E. was the principal author. Marin J.M. supervised and helped to complete the study. Gómara S. contributed to data analysis and interpretation. All participated in the preparation of the manuscript.
Conflict of InterestsThe authors declare that they have no conflict of interests related with the topic of this manuscript.
We thank the entire Sleep-Disordered Breathing Unit team, and the staff of the Translational Research Unit of the Hospital Miguel Servet, Zaragoza, for their invaluable collaboration in the organization and collection of data.
Please cite this article as: León Subías E, Gómara de la Cal S, Marin Trigo JM. Ancho de distribución eritrocitaria en apnea obstructiva del sueño. Arch Bronconeumol. 2017;53:114–119.