Chronic obstructive pulmonary disease (COPD) increases the risk of cardiovascular disease (CVD). Red blood cell distribution width (RDW) is accepted as a powerful predictor of outcomes in patients with CVD.
AimsTo study RDW in patients with COPD, and to compare the value of this measurement with clinical, echocardiographic, nutritional and laboratory status. Secondly, we aimed to determine the effect of smoking on RDW values in healthy subjects.
MethodsOne hundred and seventy-five patients with stable COPD and 210 healthy controls were enrolled in the study. Demographic, clinical, nutritional status, echocardiographic, and laboratory characteristics, RDW values were recorded and compared.
ResultsRDW values were higher in the COPD group than in controls (15±2.3% vs 13.8±2.5%, p<0.001). In COPD patients, RDW levels positively correlated with CRP levels (r=0.27, p<.001), albumin levels (r=0.23, P=.04), right ventricular dysfunction (RVD) (r=0.24, P=.001), pulmonary hypertension (PAH) (r=0.1, P=.02), and presence of CVD (r=0.24, P=.02). In multivariable logistic regression suggested that presence of CVD (4.3; 95% CI: 1.3–11; P=.01), and presence of RVD (3.1; 95% CI: 1.7–8.3; P=.02) were independently related to elevated RDW levels in COPD patients. In the healthy population, correlations analysis showed only a significant correlation between RDW and cigarette smoking years (r=0.57, p<.001).
ConclusionRDW is independently associated with CVD and RVD in patients with COPD. In the healthy population, RDW is also associated with smoking status.
La enfermedad pulmonar obstructiva crónica (EPOC) incrementa el riesgo de enfermedad cardiovascular (ECV). La amplitud de distribución eritrocitaria (ADE) se considera un potente factor de predicción de la evolución de los pacientes con ECV.
ObjetivosAnalizar los valores de ADE de pacientes con EPOC y compararlos en relación al estado clínico, ecocardiográfico, nutricional y analítico de los pacientes. Por otra parte, nos propusimos analizar el efecto del consumo de tabaco sobre los valores de ADE de sujetos sanos.
MétodosEn el estudio se incluyeron 175 pacientes con EPOC estabilizados y 210 sujetos sanos. Se registraron y se compararon las características demográficas, clínicas, nutricionales, ecocardiográficas y analíticas, y los valores de ADE.
ResultadosLos valores de ADE fueron más altos en el grupo de pacientes con EPOC que en el grupo control (15±2,3% vs 13,8±2,5%, p<0,001). Los valores de ADE de los pacientes con EPOC mostraron una correlación positiva con las concentraciones de PCR (r=0,27, p<0,001), las concentraciones de albúmina (r=0,23, P=0,04) y la presencia de disfunción ventricular derecha (DVD) (r=0,24, P=0,001), hipertensión pulmonar (HAP) (r=0,1, P=0,02) y ECV (r=0,24, P=0,02). El análisis de regresión logística para variables múltiples sugirió una relación independiente de la presencia de ECV (4,3; IC 95%: 1,3-11; P=0,01) y de DVD (3,1; IC 95%: 1,7-8,3; P=0,02) con valores elevados de ADE en los pacientes con EPOC. En la población sana, el análisis solo mostró una correlación significativa entre la ADE y la duración del consumo de tabaco (r=0,57, p<0,001).
ConclusiónEn pacientes con EPOC, la ADE se asocia de manera independiente con la ECV y la DVD. En la población sana, la ADE también se asocia con el consumo de tabaco.
Cardiovascular disease (CVD), such as congestive heart failure, hypertension, arrhythmia, and coronary artery disease, has been reported to be common in chronic obstructive pulmonary disease (COPD) patients.1–3 Therefore, COPD is known to be associated with cardiovascular morbidity and mortality.4 The most obvious explanation for the high cardiovascular morbidity and mortality rates seen in COPD patients is the high prevalence among this group of smoking and other known risk factors for CVD.4 In addition, COPD is associated with oxidative stress, and inflammation that may also initiate or worsen co-morbid diseases, such as CVD.5
Red cell distribution width (RDW) is a measure of the variability in size of circulating erythrocytes, and is routinely reported as a component of a complete blood count in the differential diagnosis of anemia.6 Recently, studies have reported that RDW might provide useful information for the prognosis of patients with CVD7–10 as well as for those with noncardiac diseases, including stroke and pulmonary hypertension, and also in the general population.11–13All these studies hypothesized that higher RDW levels might reflect an underlying chronic inflammation, which would result in an increased risk of CVD and increased mortality.
COPD also has a systemic inflammatory effect. The inflammatory process may extend beyond the pulmonary system, resulting in a state of persistent low-grade systemic inflammation which has been implicated in various complications of COPD including cachexia, CVD, and arrhythmias.5,14,15 Inflammation has been proposed as a key element in the association of COPD and CVD. It can be concluded that RDW levels, which is considered to be a marker of inflammation, may be elevated in patients with COPD as well as in CVD. Two recent studies have reported that high RDW levels in patients with COPD correlated with right ventricular dysfunction and overall survival.16,17
Smokers also have systemic inflammation.18 Clinical and experimental studies have noted that systemic markers of inflammation such as C-reactive protein (CRP), fibrinogen, interleukin-6, soluble intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 are elevated in smokers.18 Only 1 study found high RDW levels in smokers.19
Very few studies in COPD patients have measured RDW levels. In this study, we aimed to find; (1) RDW levels in COPD patients, (2) the effect of biochemical, nutritional, cardiac and respiratory parameters, and of smoking on RDW levels in patients with COPD, (3) the effect of smoking on RDW values in healthy people, and (4) the benefit of RDW, as a routinely available marker, as a predictor of CVD in COPD patients.
MethodsStudy PopulationOne hundred and seventy-five consecutive patients with stabilized COPD, admitted to hospital or 8 weeks after discharge, were enrolled in the study. The cause of the admission was routine follow-up. In addition, 110 age- and sex-matched healthy volunteers were enrolled as a control group. Subjects with a history of cancer, sleep apnea syndrome, primary valvular heart disease, connective tissue disorders, inflammatory bowel disease, renal failure, liver diseases, hematological system diseases, history of iron or vitamin deficiencies (such as B12 or folate), history of exacerbation during the last 2 months, anti-inflammatory drug (systemic steroids, immunosuppressive drugs) use in the last 2 months, and blood transfusion were excluded. The study was carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association, and was approved by the Ethics Committee of our hospital.
Demographic characteristics (age, sex, body mass index [BMI], current smoking status, history of preexisting diseases, and current drug use), and medical history, including cardiovascular and metabolic diseases, medication use, and habits were recorded (Table 1). CVD was identified if the patients had heart failure, coronary artery disease, or arrhythmia. CVD was defined by history, echocardiography, electrocardiography, and clinical evaluation by an expert cardiologist (IO).
Demographic and Spirometric Characteristics of the Study Group.
| COPD patients (n=175) | Healthy subjects (n=210) | P Value | |
|---|---|---|---|
| Age (years)a | 61±7.4 | 57.4±11 | NS |
| Gender (male), n, (%) | 110 (63) | 119 (57) | NS |
| BMI (kg/m2)a | 23.8±3.9 | 25.8±2.1 | NS |
| Smoking status | |||
| Smoking (pack-years)a | 43.4±1.1 | 25.5±1.1 | <0.05 |
| Active smoker, (n, %) | 77 (44) | 113 (54) | 0.02 |
| Ex-smoker, (n, %) | 89 (51) | 25 (12) | <0.001 |
| No smoker, (n, %) | 9 (5) | 72 (34) | <0.001 |
| Laboratory variables | |||
| RDW (%)a | 15.04±2.3 | 13.08±2.5 | <0.01 |
| Hgb (g/dL)a | 13.3±1.5 | 14.1±3.2 | NS |
| CRP (mg/dL)b | 1.1 (0.5–2.9) | 0.5 (0.4–0.7) | <0.001 |
| Albumin (g/dL)a | 3.3±0.5 | 3.5±1.2 | NS |
| PaO2 (mmHg)a | 64±11 | – | NA |
| PaCO2 (mmHg)a | 47±5.2 | – | NA |
| pHb | 7.37 (7.35–7.43) | – | NA |
| Pulmonary function tests | |||
| FVC (% predicted)b | 43 (32–60) | 96.3 (63–110) | <0.001 |
| FEV1 (% predicted)b | 36 (26–57) | 91.8 (78–105) | <0.001 |
| FEV1/FVC (% predicted)b | 64 (52–70) | 95.1 (77–98) | <0.001 |
BMI: body mass index; CRP: C-reactive protein; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; n, number of cases; NA; not available; NS: statistically nonsignificant.
RDW and hemoglobin levels were determined using the Beckman Coulter LH-750 Hematology Analyzer (Beckman Coulter, Inc., Fullerton, CA, USA), in the context of a hemogram. The normal range for RDW in our laboratory was 11.5%–15.5%. Serum albumin levels (N: 3.5–5.5g/dL) were measured using Abbot-labeled kits (catalog no: 30-3050/R2), and CRP levels (N: 0–1mg/dL) were measured by immunoturbidimetric methods in Beckman Coulter Synchron LX-20 chemistry autoanalyser in the biochemistry laboratory of our hospital. Blood gases were measured on room air (Rapid lab 348, Biobak, Chiron, Bayer Diagnostic, UK).
Pulmonary Function TestsSpirometry results, if performed during hospital stay or 8 weeks after discharge, were taken from patient records. A respiratory function test was performed with the same kind of spirometer at each center (V Max Series 20C, Sensor Medics, Yorba Linda, CA/Fukuda Denshi Spirosift 500). Spirometry was performed with equipment that met American Thoracic Society performance criteria.20 The disease severity of patients was evaluated according to Global Initiative for Chronic Obstructive Lung Disease criteria by 2 chest physicians blinded to the study dates.21
Echocardiographic FindingsEchocardiography was performed by SONOS 4500 echocardiogram using 3.5 and 2.5MHz transducers. (Sonos 5500, Philips, MA, USA). Standardized projections and measurements were performed for the evaluation of cardiac anatomy, ventricular function, and valve competence; left ventricular ejection fraction (LVEF) was measured by Simpson's method, using second harmonic imaging. Systolic pulmonary artery pressure was calculated by adding the estimated right atrial pressure to the tricuspid regurgitation gradient. Pulmonary arterial hypertension (PAH) was defined as a higher than 30mmHg systolic pulmonary artery pressure. Right ventricular dysfunction (RVD) was diagnosed in the presence of any of the followings: dilatation of the RV (diastolic diameter >30mm), abnormal motion of the interventricular septum, hypokinesis of RV, or tricuspid valve regurgitation (jet velocity, 92.5m/s).21 Left ventricular dysfunction (LVD) was defined as lower than 50% of LVEF and higher than 35mm of diastolic diameter.
Nutritional EvaluationAnthropometric and biochemical measurements were taken to assess the nutritional and biochemical status of study patients. For anthropometric measurement, BMI [weight (kg)/height (m)2] was calculated. For biochemical measurements albumin level (N: 3.5–5.5g/dL) was quantified.
Statistical AnalysesVariables are expressed as means with standard deviation or medians with interquartile range. Student's t test was used to compare means and Mann–Whitney U test was used to compare medians. Frequencies were compared with chi-square and Fisher's exact test. Spearman's and Pearson's correlation tests were used for correlation analyses. The median RDW value was calculated, and patients with COPD were classified into 2 groups, namely, those above and equal to or below the median RDW. Independent variables with P values of 0.15 from the univariate analysis were re-analyzed using multivariate logistic regression analysis. Stepwise multivariate linear regression was also performed to determine the factors independently associated with elevated RDW levels. A ROC analysis was performed to determine the RDW cut-off value for RVD.
ResultsPatients with COPD had significantly higher RDW values compared with control subjects (patients with COPD, 15.04±2.3; range, 10.4–24.5; control subjects, 13.8±2.5; range, 10–17.2; p<0.001) (Table 1) (Fig. 1). Seventy patients (40%) and 23 (11%) control subjects had an RDW>15.5%.
In COPD patients, RDW levels positively correlated with CRP levels (r=0.27, P=0.001), RVD (r=0.24, P=0.001), PAH (r=0.1, P=0.02), presence of CVD (r=0.24, P=0.002), and inversely correlated with hemoglobin (Hgb) concentration (r=−0.26, P=0.01), and serum albumin levels (r=−0.23, P=0.04) (Table 2). In healthy population, correlation analyses showed a significant correlation between RDW and the cigarette pack years (r=0.57, p<0.001), and inversely correlated with Hgb concentration (r=−0.38, P=0.01) (Table 2).
Correlation Coefficients Between Demographic and Laboratory Variables and RDW.
| Variable | COPD Patients | Healthy Subjects | ||
|---|---|---|---|---|
| RDW | RDW | |||
| r Coefficient | P Value | r Coefficient | P Value | |
| Demographic characteristics | ||||
| Mean age (years)a | 0.09 | NS | −0.1 | NS |
| Gender (male, n, %) | −0.06 | NS | −0.2 | NS |
| Pack-years of smokinga | −0.04 | NS | 0.57 | <0.001 |
| Active smoker, (n, %) | 0.09 | NS | 0.18 | NS |
| Ex-smoker, (n, %) | 0.05 | NS | 0.04 | NS |
| BMI (kg/m2)a | 0.02 | NS | −0.2 | NS |
| Respiratory findings | ||||
| FEV1 (%)b | 0.07 | NS | 0.06 | NS |
| FEV1/FVC (% predicted)b | 0.06 | NS | 0.1 | NS |
| FVC (%)b | −0.01 | NS | −0.1 | NS |
| Comorbidity | NA | |||
| Hypertension (n, %) | −0.03 | NS | ||
| Diabetes mellitus (n, %) | 0.1 | NS | ||
| CVD (n, %) | 0.24 | 0.002 | ||
| Laboratory variables | ||||
| Hgb (g/dL)a | −0.26 | <0.001 | −0.38 | 0.01 |
| CRP (mg/dL)b | 0.27 | <0.001 | −0.09 | NS |
| Albumin (g/dL)a | −0.23 | 0.04 | 0.18 | NS |
| PaO2 (mmHg)a | −0.07 | NS | NA | |
| PaCO2 (mmHg)a | 0.16 | NS | NA | |
| pHb | 0.05 | NS | NA | |
| Echocardiographic parameters | NA | |||
| LVD (n, %) | −0.05 | NS | ||
| RVD (n, %) | 0.24 | <0.001 | ||
| Presence of PAH (n, %) | 0.1 | 0.02 | ||
BMI: body mass index; CRP: C-reactive protein; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; LVD: left ventricular dysfunction; n: number of cases; NA: not available; NS: not statistically significant; PAH: pulmonary arterial hypertension; RVD: right ventricular dysfunction.
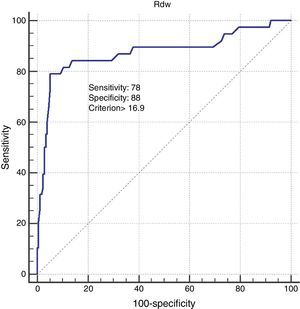
Comparing patients with elevated (>15.5%) RDW values and nonelevated (≤15.5%) RDW values showed that the former were more likely to have higher CRP and PaCO2 levels, and lower FEV1 levels. In addition RVD, PAH, and presence of CVD were significantly higher in patients with elevated RDW (Table 3). In the healthy population, RDW values were significantly higher in smokers compared to non-smokers (Table 4). Additionally, in healthy subjects, only pack-year of smoking was statistically significant between those with high RDW levels and not. In COPD patients, univariate analysis showed elevated (>15.5%) RDW values were most closely associated with higher CRP levels (P=0.001), higher PaCO2 levels (P=0.02), lower Hgb levels (P=0.01), presence of PAH (P=0.1), presence of CVD (P=0.001), and presence of RVD (P=0.004). In the multivariate analysis, patients with an elevated RDW value were significantly more likely to have presented CVD (P=0.01), and RVD (P=0.02) (Table 5). ROC analysis showed 16.9 RDW value is as optimum cut-off value for diagnosis of RVD. When using this cut-off value, the sensitivity and specificity of RDW in diagnosis of RVD was 78% and 88.7%, respectively (Fig. 2).
Evaluation of Parameters Between Groups With Normal RDW and Higher RDW in COPD Patients.
| RDW>15.5% (n=70) | RDW≤15.5% (n=105) | P Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age (years)a | 61.7±7.5 | 60.3±7.4 | NS |
| Gender (male, n, %) | 47 (68) | 63 (60) | NS |
| Pack-years of smokingb | 40 (10–60) | 38 (8–54) | NS |
| Active smoker, (n, %) | 28 (40) | 49 (46) | NS |
| Ex-smoker, (n, %) | 35 (50) | 54 (46) | NS |
| BMI (kg/m2)a | 26.5±6.1 | 26±4.6 | NS |
| Respiratory findings | |||
| FEV1 (%)b | 33 (20–70) | 37 (20–70) | <0.05 |
| FEV1/FVC (% predicted)b | 67 (56–74) | 68 (56–75) | NS |
| FVC (%)b | 41 (30–54) | 43 (34–67) | NS |
| Comorbidity | |||
| Hypertension (n, %) | 15(20) | 20 (19) | NS |
| Diabetes mellitus (n, %) | 4 (5) | 8 (5) | NS |
| CVD (n, %) | 18 (24) | 12 (11) | 0.001 |
| Blood parameters | |||
| Hemoglobin (g/dL)a | 13.2±2.1 | 14.1±3.2 | 0.011 |
| MCV (fL)a | 83.7±4.1 | 87±7.2 | <0.001 |
| CRP (mg/dL)b | 2.7 (0.7–10) | 1.5 (0.6–3.8) | <0.001 |
| Albumin (g/dL)a | 3.3±0.5 | 3.6±1.2 | 0.04 |
| PaO2 (mmHg)a | 65±10 | 68±9 | NS |
| PaCO2 (mmHg)a | 47±8.6 | 43±6.3 | <0.05 |
| pHb | 7.39 (7.35–7.44) | 7.4 (7.37–7.42) | NS |
| Time since onset of disease (years)b | 14 (7–20) | 12 (6–20) | NS |
| Echocardiographic parameters | |||
| LV dysfunction (n, %) | 6 (8) | 11(10) | NS |
| RV dysfunction (n, %) | 32(42) | 26(25) | 0.001 |
| Presence of PAH (n, %) | 43 (57) | 42 (40) | 004 |
BMI: body mass index; CRP: C-reactive protein; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; LVD: left ventricular dysfunction; MCV: mean corpuscular volume; n: number of cases; NS, not statistically significant; PAH: pulmonary arterial hypertension; RVD: right ventricular dysfunction; p-value: elevated versus non-elevated RDW.
Baseline Characteristics, Spirometric and Blood Parameters of Healthy Subjects.
| Smokers (n=113) | Non-smokers (n=72) | P Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age (years)a | 55±7.3 | 52.7±3 | NS |
| Gender (male, n, %) | 59 (52) | 40 (55) | NS |
| BMI (kg/m2)a | 23.5±3.1 | 25.5±7 | NS |
| Respiratory findings | |||
| FEV1 (% predicted)b | 94.1(56–110) | 97.5 (53–105) | NS |
| FEV1/FVC (% predicted)b | 87.8 (78–105) | 91 (80–105) | NS |
| FVC (% predicted)b | 95.1 (77–98) | 92.3 (80–98) | NS |
| Blood parameters | |||
| RDWa | 13.09±1.3 | 11.04±2.3 | 0.002 |
| Hemoglobin (g/dL)a | 13.4±2.1 | 14.6±3.2 | NS |
| MCV (fL)a | 83.7±4.1 | 87±7.2 | <0.05 |
| CRP (mg/dL)b | 0.95 (0.7–4.6) | 1.3 (0.6–5.8) | NS |
| Albumin (g/dL)a | 3.3±0.5 | 3.6±1.1 | NS |
BMI: body mass index; CRP: C-reactive protein; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; LVD: left ventricular dysfunction; MCV: mean corpuscular volume; n: number of cases; NS, not statistically significant; PAH: pulmonary arterial hypertension; RVD: right ventricular dysfunction; p-value: elevated versus non-elevated RDW.
Univariate and Multivariate Analysis of Risk Factors for Elevated RDW Levels in Patients With COPD.
| Variables | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| p-Value | p-Value | |
| OR (95% CI) | OR (95% CI) | |
| Age | 0.17 | |
| 1.026 (0.98–1) | ||
| Gender | 0.98 | |
| 1 (0.5–1.9) | ||
| BMI | 0.6 | |
| 0.99 (0.91–1.18) | ||
| Pack-years of smoking | 0.69 | |
| 1 (0.9–1) | ||
| Active smoker | 0.75 | |
| 0.8 (0.3–1.9) | ||
| CRP | <0.001 | 0.7 |
| 1.3 (1.1–1.4) | 0.9 (0.4–1.4) | |
| PaO2 | 0.23 | |
| 0.9 (0.95–1.01) | ||
| PaCO2 | 0.04 | 0.11 |
| 1.04 (1.01–1.91) | 1.04 (0.98–1.1) | |
| Albumin | <0.05 | 0.52 |
| 0.36 (0.17–0.75) | 0.9 (0.86–1) | |
| Hemoglobin | 0.004 | 0.06 |
| 0.8 (0.6–0.9) | 0.8 (0.6–1.01) | |
| FVC, % predicted | 0.18 | |
| 1.6 (0.9–1.1) | ||
| FEV1/FVC, % predicted | 0.45 | |
| 1.01 (0.97–1.06) | ||
| FEV1, % predicted | 0.9 | |
| 0.97 (0.95–1) | ||
| Presence of PAH | 0.08 | 0.5 |
| 0.6 (0.3–1.1) | 0.74 (0.3–1.8) | |
| Presence of CVD | <0.001 | 0.01 |
| 5.3 (2.7–9.4) | 4.3 (1.3–11) | |
| Presence of RVD | <0.001 | 0.02 |
| 6.08 (3.1–11.8) | 3.1 (1.7–8.3) | |
| Presence of LVD | 0.46 | |
| 0.58 (0.14–2.45) | ||
| Presence of hypertension | 0.44 | |
| 1.3 (0.6–3.2) |
BMI: body mass index; CRP: C-reactive protein; CVD: cardiovascular disease; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; LVD: left ventricular dysfunction; PAH: pulmonary arterial hypertension; RVD: right ventricular dysfunction.
In the last few years, numerous studies have shown that RDW is a strong prognostic factor for all-cause mortality and CVD events in patients with a history of heart disease or chronic heart failure.7–10 In addition, RDW is strong predictor of all-cause mortality in population cohorts.10,11,13 All these studies suggest that the pathophysiology leading to increased RDW may reflect underlying chronic inflammation, which would result in an increased risk of CVD and increased mortality.5,14,15 We hypothesized that a chronic inflammatory state may be a mechanistic link between higher RDW and in COPD patients with CVD. In the present study, we have shown that COPD patients have elevated levels of RDW compared to controls. An addition, RDW was found to be an independent predictor of CVD in COPD patients.
Increased RDW is the result of heterogeneity of erythrocyte size and erythrocyte fragmentation in the circulation.6 Factors that contribute to increased erythrocyte size heterogeneity include iron or vitamin B12, folate deficiency, decreased erythrocyte life span, impaired erythropoiesis, or blood transfusion.6 Inflammation can also elevate RDW levels.22,23 Very recently, studies have shown that increased RDW values are also associated with adverse outcomes in CVD as well as noncardiovascular disease states, even in patients with heart failure, pulmonary hypertension, pulmonary embolism, sleep apnea, and in patients in the intensive care unit.7,9,12,13,24–26 Elevated RDW levels have also been strongly associated with increased risk of death and cardiovascular disease (CVD) events in middle-aged and older adults.13,27 In the 2 studies focusing on RDW levels in COPD published to date,16,17 the authors concluded that RDW could be a marker of long-term prognosis and RVD. We found in our study that RDW levels were significantly higher in patients with COPD compared to healthy subjects. Our primary goal was not to discriminate COPD patients from healthy subjects. However, the discriminative capacity is not high when comparing COPD patients and healthy subjects. In line with Dincer et al.,17 we found high RDW levels to be associated with CVD in patients with COPD.
CVD is more common among patients with COPD than in the general population.4 It is also a major risk factor for the development of CVD. Additionally, hypoxemia, oxidative stress and systemic inflammation are thought to contribute to the development of CVD in COPD.4,5 Through all these pathways, COPD has a potential link with CVD from the point of view of inflammation, atherosclerosis, and endothelial dysfunction.28 Inflammatory cytokine release in COPD could affect bone marrow function, and erythropoietin-induced erythrocyte maturation is inhibited, thus elevating RDW.22 Therefore, higher RDW levels might reflect an underlying chronic inflammation, which would result in an increased risk of CVD in COPD patients. In the present study, we found that CRP and RDW are significantly correlated. This finding may show a possible link between inflammation in COPD and RDW values. Co-existing CVD was detected in 34% of COPD patients in this study. We also concluded that the primary cause of high RDW levels in COPD patients was co-existing CVD.
Pulmonary hypertension (PH) leads to right ventricular hypertrophy, right ventricular enlargement, and finally to RVD. Hampole et al. investigated the relationship between RDW and mortality in 162 patients with PH, and found that RDW was independently associated with death in patients with PH, and that it performs better as a prognostic indicator than N-terminal pro-natriuretic peptide.12 A different study in COPD showed that high RDW levels were associated with RVD.17 In Dincer's study, 17.7 was established as the cut-off value for detecting RVD in COPD patients. In line with these studies, we concluded that high RDW is closely correlated with PH and RVD in COPD patients. Additionally, we found that an RDW level of 16.9 is highly sensitive and specific for the diagnosis of RVD, and that – by multivariate analysis – RVD was one of 2 factors influencing RDW levels in patients with COPD. We believe that high RDW value may be an indicator of RVD.
Smoking is the leading risk factor for COPD. It is also a major risk factor for the development of CVD.4 Smoking itself may cause systemic inflammation and, for example, increased total leukocyte count, but in COPD patients the degree of systemic inflammation is higher.18 Local inflammation is characterized by increased numbers of inflammatory cells, such as neutrophils, lymphocytes, and macrophages, and higher TNF-α and IL-8 concentrations in smokers than healthy controls.29 These results suggest that smoking may be associated with higher CRP production in the liver of subjects, and also with mechanisms related to systemic inflammation. A recent study19 exploring the association between smoking and RDW levels in healthy subjects showed that smoking raised RDW levels, and duration of smoking habit along with the number of pack-years correlated highly with RDW levels. In the present study, we detected a positive correlation between pack-years and RDW in healthy subjects. Additionally, RDW values were significantly higher in smokers compared to non-smokers. This finding suggests that systemic inflammation may also occur in healthy smokers who do not develop COPD. Therefore, we hypothesized that elevated RDW is associated with smoking, and may be a useful indicator of inflammatory activity in healthy smokers.
This study has some limitations: the sample size is small, and some correlations are weak. A longitudinal study to determine a causative relationship between systemic inflammation and CVD is needed to confirm our findings. This paper should be considered a pilot study needing a further validation step.
In conclusion, elevated RDW may be an indicator of underlying inflammation in COPD, and may in itself be a useful and simple tool to evaluating CVD and RVD in COPD patients. RDW was found to be more sensitive than CRP in predicting right ventricular dysfunction and cardiovascular disease in COPD patients. Additionally, measurement of RDW is more widely available and less expensive than measurement of other inflammatory cytokines (TNF-α, IL-8, etc.). RDW may also be a biomarker of systemic inflammation in smoking in healthy people.
AuthorshipDr. ECS performed all the procedures, prepared the database and drafted the manuscript.
Dr. GO assisted Dr. ECS in performing the procedures, organized the database and contributed to the drafting of the manuscript.
Dr. MAO assisted Dr. ECS in performing the procedures and preparing the database.
Conflict of InterestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Ozgul G, Seyhan EC, Ozgul MA, Gunluoglu MZ. Amplitud de distribución eritrocitaria en pacientes con enfermedad pulmonar obstructiva crónica y en sujetos sanos. Arch Bronconeumol. 2017;53:107–113.









![The RDW values were higher in the COPD group than in the controls [15.04±2.3 vs 13.08±2.5, P=0.01]. The RDW values were higher in the COPD group than in the controls [15.04±2.3 vs 13.08±2.5, P=0.01].](https://static.elsevier.es/multimedia/15792129/0000005300000003/v1_201703240112/S1579212916301355/v1_201703240112/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)