Interstitial lung disease (ILD) is one of the major causes of death in systemic sclerosis (SSc). This study investigated exhaled breath (EB) and exhaled breath condensate (EBC) biomarkers in patients with SSc and analyzed their role as a prognostic tool in SSc-related ILD.
MethodsFraction exhaled nitric oxide (FeNO) and exhaled carbon monoxide (eCO) measured in EB, together with pH, nitrite, nitrate and interleukin-6 levels measured in EBC were prospectively analyzed in 35 patients with SSc. Twelve patients had established ILD by chest high-resolution computed tomography (HRCT), and 23 patients showed no evidence of ILD. EB and EBC biomarkers were determined at inclusion, and pulmonary function tests were annually performed during 4 years of follow-up.
ResultsNo differences at baseline biomarkers levels were found between groups. In all patients studied, low EBC pH levels were associated with a decreased diffusing capacity for carbon monoxide (DLCO) during follow-up. Low FeNO levels were correlated with lower forced vital capacity (FVC) at baseline, 4 years of follow-up and with a decrease in FVC and DLCO during monitoring. Among ILD patients, high eCO levels were correlated with lower baseline FVC. In the global cohort, a worse progression-free survival was identified in patients with EBC pH values lower than 7.88 and FeNO levels lower than 10.75ppb (log Rank P=0.03 and P<0.01, respectively).
ConclusionsEB and EBC could help to detect patients likely to present a deterioration on lung function during follow up.
La enfermedad pulmonar intersticial (EPI) es una de las principales causas de muerte en los pacientes con esclerosis sistémica (ES). En este estudio se investigaron biomarcadores en el aire exhalado (AE) y en el condensado de aire exhalado (CAE) y se analizó su posible papel como factores pronóstico de la EPI en pacientes con ES.
MétodosSe analizó prospectivamente la fracción exhalada de óxido nítrico (FeNO) y el monóxido de carbono exhalado (COe) en AE, y se determinaron los valores de pH, nitritos, nitratos e interleucina-6 en CAE, en 35 pacientes con ES. La tomografía computarizada de alta resolución (TACAR) torácica mostró signos de EPI en 12 pacientes, no estando presentes en los 23 restantes. En el momento de la inclusión se determinaron los biomarcadores en el AE y en el CAE, y durante los 4 años de seguimiento se efectuaron anualmente pruebas de función respiratoria.
ResultadosNo se observaron diferencias entre grupos en los valores iniciales de los diferentes biomarcadores. En todos los pacientes examinados los valores disminuidos de pH en CAE se asociaron con una reducción en la capacidad de difusión de monóxido de carbono (DLCO) durante el seguimiento. Valores disminuidos de FeNO se correlacionaron con una menor capacidad vital forzada (FVC) inicial y a los 4años, así como con una reducción de FVC y DLCO durante el seguimiento. En los pacientes con EPI los valores más altos de COe se correlacionaron con FVC más disminuidas al inicio. En el conjunto de la cohorte se identificó una menor supervivencia libre de progresión en los pacientes con un pH en CAE inferior a 7,88 y en los que presentaban un FeNO inferior a 10,75ppb (Log Rank: p=0,03 y p<0,01, respectivamente).
ConclusionesLos biomarcadores en el AE y en el CAE son útiles para detectar pacientes con una mayor probabilidad de presentar un deterioro de la función pulmonar durante el seguimiento de la enfermedad.
Systemic sclerosis (SSc) is a connective tissue disease characterized by small-vessel vasculopathy, fibroblast dysfunction with excessive collagen production, fibrosis, and immunological abnormalities.1 Currently, interstitial lung disease (ILD) is one of the leading causes of SSc-related deaths.2,3 The prevalence of SSc-associated ILD varies depending on the population studied, case definition, and sensitivity of diagnostic methods. Goh et al.4 proposed a simple stratification scheme based on the extent of disease on high resolution computed tomography (HRCT) and the impact on pulmonary function testing (PFT), which yielded the best prediction of disease progression and long-term mortality. Recently, serum interleukin-6 (IL-6) has been reported as a predictor of SSc-ILD progression, specifically in patients with early-mild stages.5
An increase in inflammatory cells on bronchoalveolar lavage (BAL) is a common feature in SSc-related ILD.6,7 However, there is no correlation with long-term survival or progression,8 therefore it is only used to exclude an infection and for research purposes.3 Although the study of several biomarkers in induced sputum, exhaled breath (EB) and exhaled breath condensate (EBC) has been reported to be useful in scleroderma pulmonary involvement,9–13 their potential value is still controversial, and additional non-invasive biomarkers are needed to predict disease progression.
We sought to demonstrate that SSc-related ILD might be mediated by an autoimmune inflammatory process in the lung, causing a release of cytokines and metabolites that could be measured non-invasively in EB and/or EBC. This study had 2 main objectives: first, to analyze EB and EBC biomarkers in scleroderma patients based on the presence of ILD, and study their association with baseline PFTs; and secondly, to determine whether these biomarkers correlate with disease progression at 4 years, having a potential prognostic significance.
MethodsPatientsA prospective study was performed including 35 Caucasian patients (32 women) diagnosed with SSc. All patients fulfilled the ACR/EULAR 2013 classification criteria,14 had undergone a baseline HRCT, and were followed-up for 4 years. Patients were consecutively assessed in the Scleroderma Unit at our hospital and followed up by the same specialist every 4 months in moderate-severe ILD patients, or every 6 months in patients with no pulmonary involvement or mild ILD, following international guidelines.15 The study was approved by the hospital's Ethics Committee for Clinical Research, and all the patients provided informed consent for their participation, according to the Declaration of Helsinki. The exclusion criteria were the presence of other inflammatory respiratory diseases (such as asthma, COPD or others), atopic features at inclusion, or an infection of the respiratory tract during the previous month.
Study GroupsThe study population was divided into 2 groups: the ILD group included 12 patients with ILD defined as radiological evidence of interstitial disease on HRCT, containing 1 patient with pulmonary hypertension associated to ILD (PH-ILD); and 23 SSc patients with no evidence of ILD were selected as the control group, including 2 patients with isolated pulmonary arterial hypertension (PAH). PH was defined as mean pulmonary arterial pressure (mPAP) ≥25mmHg in right heart catheterization (RHC). PH-ILD was considered if PH was diagnosed in a patient with ILD and lower than 60% predicted forced vital capacity (FVC) or moderate-severe interstitial disease in the HRCT.16 Isolated PAH was diagnosed by RHC according to international guidelines.16,17 Other SSc comorbidities were also recorded as previously described.18 All patients underwent complete PFTs, fraction of exhaled NO (FeNO), exhaled CO (eCO) and EBC collection at baseline. Subjects were instructed to refrain from food intake and smoking during the 2 and 24 h prior to sample collection, respectively. All samples were analyzed in the same laboratory.
Pulmonary Function TestsSpirometry, static lung volumes and transfer lung studies were performed annually using MasterLab equipment (MasterLab, Jaegger, Germany) following the ERS-ATS recommendations.19 The PFTs outcomes were analyzed as the change in % predicted FVC or diffusing capacity for carbon monoxide (DLCO) compared to baseline.
Fraction of Exhaled NO MeasurementFeNO measurements were performed using NIOX MINO® device (Aerocrine AB, Solna, Sweden) according to current guidelines.20 Patients sat in an upright position and wore a nose clip during the process. Patients inhaled NO-free air through the device and subsequently exhaled at a fixed flow rate of 50mL/s for approximately 10s. The first technically acceptable FeNO measurement was used for analysis.21
Exhaled CO MeasurementExhaled CO was measured by a MicroCO Meter (MicroCO – Micro Medical Ltd, Rochester, Kent, UK), using an electrochemical sensor. Patients were instructed to breath deeply to total lung capacity, to hold the breath for 20s, and then to exhale slowly and completely through a mouthpiece. Two successive recordings were made and maximal values were used in all calculations.
EBC Samples Collection and ProcessingEBC was collected during tidal breathing with a commercially available condenser (EcoScreen; Jaeger, Würzburg, Germany). Subjects breathed tidally through a mouthpiece connected to the condenser while wearing nose clips. A fixed volume of 150L of exhaled breath was collected per subject.22 EBC samples were processed in sampling tubes and divided into 500μL aliquots. One aliquot was used to measure the pH after deaeration, and the remaining were immediately stored at −70°C, and analyzed within 1 month of collection.
EBC pH was measured after deaeration with helium (350mL/min for 10min), using a calibrated pH meter (Model GLP 21; Crison Instruments SA; Barcelona, Spain) with an accuracy of ±0.01 pH, and a probe for small volumes (Crison 50 28; Crison Instruments SA; Barcelona, Spain). The probe was calibrated daily with standard pH 7.02 and 4.00 buffers.23
EBC nitrite (NO2−) and nitrate (NO3−) levels were determined with a colorimetric assay based on the Griess reaction (Cayman Chemical Company, USA). The concentrations were measured at 540nm absorbance with a microplate reader. Assay sensitivity was 1μM for nitrite and 2.5μM for nitrate.
IL-6 levels in EBC samples were evaluated by a commercially available high sensitivity enzyme-linked immunosorbent assay (ELISA) (Bender MedSystems GmbH, Austria). Assay sensitivity was 0.03pg/mL.
Statistical AnalysisNon-parametric tests were performed to assess the presence of statistically significant differences. Categorical data were analyzed by chi-square and Fisher's exact tests. Continuous data were analyzed using the Mann–Whitney U test in the between-groups differences and Wilcoxon signed rank test in within-group comparisons. Associations between variables were analyzed using Spearman's correlation. Receiver Operating Characteristic (ROC) curves and Youden's index method were used to determine the threshold value of each biomarker, which demonstrated the best sensitivity and specificity to predict a decrease of 10% in FVC, a 15% in DLCO or death. Log Rank test was used to measure the progression-free survival (PFS) in the follow-up, whose endpoint was the occurrence of combined damaging events, defined as a 10% decrease in FVC, a 15% decrease in DLCO from baseline, or death. A P value <0.05 was considered significant. SPSS version 20.0 for Windows (SPSS Inc., Chicago) and GraphPad InStat4 (GraphPad Software Inc., San Diego) were used to carry out the statistical analyses.
ResultsBaseline DataDemographic data and baseline characteristics of the study population are shown in Table 1. Median (interquartile range, IQR) age was 59.0 (42.0 to 68.0) years. HRCT was consistent with non-specific interstitial pneumonia (NSIP) in all ILD patients, whereas no ILD findings were observed in controls. The median of pulmonary disease duration was 1.4 years in the ILD group. At inclusion, 7 patients were taking immunosuppressant therapy.
Demographic Variables and Baseline Characteristics of the Patients Included in the Study.
| Global (n=35) | ILD (n=12) | Controls (n=23) | P-value | |
|---|---|---|---|---|
| Female | 32 (91.4) | 10 (83.3) | 22 (95.7) | 0.26 |
| Age, years | 59.0 (42.0 to 68.0) | 64.5 (52.5 to 70.2) | 58.0 (36.0 to 67.0) | 0.31 |
| Current smoker | 4 (11.4) | 1 (8.3) | 3 (13.0) | 1.0 |
| LcSSc | 28 (80.0) | 8 (66.7) | 20 (87.0) | 0.20 |
| Time from first symptom, years | 13.0 (3.5 to 28.3) | 10.2 (1.5 to 35.4) | 13.3 (5.0 to 28.3) | 0.69 |
| Time from first non-RP symptom, years | 7.1 (1.5 to 12.6) | 7.6 (1.7 to 10.0) | 7.1 (1.5 to 13.3) | 0.71 |
| ANA | 35 (100) | 12 (100) | 23 (100) | NA |
| ACA | 18 (51.4) | 5 (41.7) | 13 (56.5) | 0.40 |
| Scl-70 | 4 (11.4) | 3 (25.0) | 1 (4.3) | 0.10 |
| ILD | 12 (34.2) | 12 (100) | 0 (0) | <0.001 |
| Disease duration ILD, years | – | 1.4 (0.3 to 5.0) | – | NA |
| PH | 3 (8.6) | 1 (8.3) | 2 (8.6) | 1.0 |
| PH-ILD | 1 (2.9) | 1 (8.3) | 0 (0) | 0.34 |
| Isolated PAH | 2 (5.7) | 0 (0) | 2 (8.6) | 0.53 |
| Gastrointestinal involvement | 32 (91.4) | 11 (91.7) | 21 (91.3) | 1.0 |
| Esophageal dysmotility | 30 (85.7) | 11 (91.7) | 19 (82.6) | 0.64 |
| Gastric involvement | 7 (20.0) | 2 (2.4) | 5 (21.7) | 1.0 |
| Cardiac involvement | 13 (37.1) | 6 (50.0) | 7 (30.4) | 0.29 |
| Renal chronic failure | 1 (2.9) | 0 (0) | 1 (4.3) | 1.0 |
| Arterial hypertension | 10 (28.6) | 3 (25.0) | 7 (6.6) | 1.0 |
| Dyslipidemia | 2 (5.7) | 1 (8.3) | 1 (4.3) | 1.0 |
| Diabetes mellitus | 0 (0) | 0 (0) | 0 (0) | NA |
| Immunosuppressant therapy | 7 (20) | 5 (41.7) | 2 (8.7) | 0.03 |
| Prednisone ≤5mg/day | 5 (14.3) | 3 (25.0) | 2 (8.7) | 0.31 |
| Sodium mycophenolate | 4 (11.4) | 3 (25.0) | 1 (4.3) | 0.10 |
| IV Cyclophosphamide | 1 (2.9) | 1 (8.3) | 0 (0) | 0.34 |
| Non-immunosuppressant drugs | 35 (100) | 12 (100) | 23 (100) | 1.0 |
| Proton pump inhibitors | 35 (100) | 12 (100) | 23 (100) | 1.0 |
| Calcium channel blockers | 22 (62.9) | 7 (58.3) | 15 (65.2) | 0.72 |
| Antiplatelet therapy | 15 (42.9) | 6 (50.0) | 9 (39.1) | 0.72 |
| ACEI or ARB | 10 (28.6) | 1 (8.3) | 9 (39.1) | 0.11 |
| N-acetylcysteine | 2 (5.7) | 2 (16.7) | 0 (0) | 0.11 |
| NO donors therapy | 2 (5.7) | 0 (0) | 2 (8.7) | 0.53 |
Data shown as median (interquartile range, IQR) for continuous variables and n (%) for categorical variables. ACA: anticentromere antibodies; ACEI: angiotensin converting enzyme inhibitors; ANA: antinuclear antibodies; ARB: angiotensin II receptor blockers; lcSSc: limited systemic sclerosis; ILD: interstitial lung disease; IV: intravenous; NA: not applicable; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; RP: Raynaud's phenomenon.
Baseline imaging, PFTs and biomarkers are described in Table 2. Significant differences at baseline were found between the study groups in the FVC, TLC, DLCO and KCO, with lower values in the ILD group. EB and EBC biomarkers were analyzed in both groups of patients at baseline. No statistical differences were found in FeNO or eCO levels. However, FeNO was positively associated with baseline FVC (r=0.41, P=0.03). In the ILD group, a negative correlation was found between eCO levels and baseline FVC (r=−0.75, P<0.01). No significant differences in EBC biomarkers were found between the 2 study groups. However, analyzing 11 patients with ground-glass opacities in the HRCT, the median of EBC pH was significantly lower than in patients without this radiographic pattern (7.4 vs. 8.0, P=0.02). No other association was observed in terms of HRCT findings with baseline biomarkers.
HRCT, PFTs, Echocardiography, Exhaled Breath and Exhaled Breath Condensate Biomarkers at Baseline.
| ILD (n=12) | Controls (n=23) | P-value | |
|---|---|---|---|
| HRCT findings | 12 (100) | 0 (0) | <0.001 |
| Ground-glass opacities | 11 (91.7) | 0 (0) | <0.001 |
| Reticular opacities | 6 (50.0) | 0 (0) | <0.01 |
| Honeycombing | 1 (8.3) | 0 (0) | 0.36 |
| Pulmonary function test | |||
| FVCa | 73.3 (62.3 to 91.9) | 90.9 (83.7 to 103.0) | 0.03 |
| FEV1a | 81.2 (65.7 to 108.4) | 98.9 (85.2 to 107.9) | 0.09 |
| FEV1% (FEV1/FVC) | 80.9 (76.1 to 88.3) | 80.6 (76.0 to 83.2) | 0.13 |
| TLCa | 82.7 (70.9 to 92.6) | 104.3 (95.8 to 111.5) | 0.001 |
| DLCOa | 47.7 (41.2 to 59.1) | 70.9 (59.1 to 86.6) | <0.01 |
| KCOa | 55.2 (46.9 to 66.5) | 67.3 (63.6 to 85.3) | 0.02 |
| Echocardiography | |||
| RVSP, mm Hg | 35.0 (29.5 to 45.0) | 31.0 (25.5 to 41.2) | 0.60 |
| Exhaled breath | |||
| FeNO, ppb | 10.5 (7.5 to 16.5) | 12.0 (9.2 to 20.7) | 0.23 |
| eCO, ppm | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 8.2) | 0.77 |
| Exhaled breath condensate | |||
| pH | 7.5 (6.9 to 7.9) | 8.0 (7.4 to 8.2) | 0.05 |
| Nitrite, μM | 3.7 (1.9 to 5.8) | 3.2 (1.4 to 4.8) | 0.57 |
| Nitrate, μM | 6.4 (3.5 to 10.9) | 8.7 (5.8 to 13.8) | 0.38 |
| IL-6, pg/mL | 0.06 (0.03 to 0.10) | 0.07 (0.05 to 0.10) | 0.61 |
DLCO: carbon monoxide diffusing capacity; eCO: exhaled carbon oxide; FeNO, fraction of exhaled nitric oxide; FEV1: forced expiratory volume in first second; FEV1%: FEV1/FVC ratio expressed as percentage; FVC: forced vital capacity; HRCT: high resolution computed tomography; IL-6: interleukin-6; ILD: interstitial lung disease; KCO: transfer coefficient; NA: not applicable; RVSP: right ventricular systolic pressure; TLC: total lung capacity.
A substudy excluding the 7 patients taking immunosuppressive therapies found the same differences in baseline PFTs and EB and EBC biomarkers.
Follow-up DataA patient from the control group with baseline EBC pH of 8.09 and FeNO of 14.5ppb developed mild ILD diagnosed by HRCT with FVC higher than 70% at 45 months of follow-up, and thereafter presented PH-ILD identified by RHC at 47 months of follow-up. Four patients died during follow-up, 2 from the ILD group and 2 controls: one due to PAH and due to a stroke.
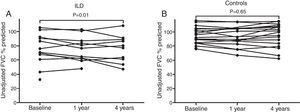
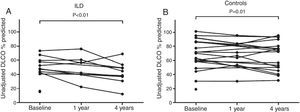
Figs. 1 and 2 show FVC and DLCO values, respectively, in the 2 study groups during the follow-up period.
FVC values at baseline and during follow-up. A and B show the unadjusted variation of % predicted FVC during follow up (each point represents a patient) for ILD patients and controls, respectively. In the ILD group, we observed a decrease of 6.4% in the mean unadjusted FVC % predicted values during the 4-year period compared to baseline (95% CI: −11.2 to −1.6; P=0.01) (A), whereas no significant differences were found in the control group (B). A higher decline of FVC % was found in ILD group than controls (P<0.01).
DLCO values at baseline and during follow-up. A and B show the unadjusted variation of % predicted DLCO during follow up (each point represents a patient) for ILD patients and controls, respectively. DLCO values were significantly lower at 4 years of follow-up compared to baseline in the ILD group, with a mean decrease of 11.1% (95% CI: −18.0 to −4.3; P<0.01) (A), and in controls, with a mean decline of 5.5% (95% CI: −10.0 to −1.1; P=0.01) (B).
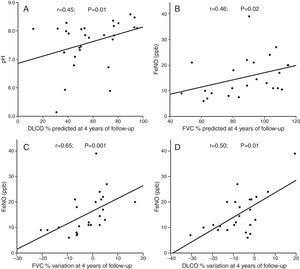
Correlations between EBC biomarkers and PFTs are shown in Fig. 3. In all patients, low pH values were correlated with lower DLCO at 4 years of follow-up (Fig. 3A). Furthermore, low FeNO levels were associated with lower FVC at 4 years of follow-up (Fig. 3B). Low baseline FeNO levels were related to a decrease of FVC and DLCO at 4 years of follow-up (Fig. 3C and D). In ILD patients, a negative correlation was found between high eCO levels and lower FVC at the end of follow-up (r=−0.66; P=0.03). In addition, we observed a trend towards correlation between high IL-6 levels and lower DLCO at end of study in SSc-ILD patients (r=−0.53; P=0.10). There were no statistical differences in the associations of the remaining EBC biomarkers.
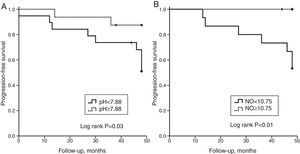
ROC curves were used to quantify the accuracy of each baseline biomarker to predict functional decline or death. The area under the curve (AUC) for EBC pH was 0.65 (95% CI: 0.41 to 0.89; P=.23), and a threshold value of 7.88 was established using Youden's index method, with a sensitivity of 0.71 and a specificity of 0.58. AUC for FeNO was 0.81 (95% CI: 0.65 to 0.96; P=0.01), and showed a threshold of 10.75ppb, with a sensitivity and specificity of 0.85 and 0.77, respectively. Kaplan–Meier curves illustrate the PFS according to the former cut-off values of pH and FeNO (Fig. 4). EBC pH lower than 7.88 showed a decrease in lung function parameters or death during follow-up (log Rank P=0.03). FeNO levels lower than 10.75ppb were related to worse PFS (log Rank P<0.01). There were no statistical differences in survival related to baseline eCO levels.
Kaplan–Meier survival plots representing progression-free survival since EBC collection. The end point was defined as a decrease from baseline FVC of at least 10%, or a 15% decrease in DLCO, or death during follow-up. (A) The survival plot found a better progression-free survival in patients with EBC pH values equal to or higher than 7.88. (B) The survival plot showed a higher progression-free survival in patients with FeNO levels equal to or greater than 10.75ppb.
In the substudy excluding patients taking immunosuppressive therapies, the AUC for EBC pH was 0.66 (95% CI: 0.44 to 0.88; P=0.18), with the same threshold value of 7.88, although there were no statistical differences in PFS (log Rank P=0.09). Regarding FeNO assays, the AUC was 0.88 (95% CI: 0.74 to 1.0; P=0.18), with a threshold of 10.25ppb, with a sensitivity and specificity of 0.80 and 0.84, respectively. Furthermore, worse PFS was identified in patients with FeNO levels under 10.25ppb (log Rank P<0.01).
DiscussionIn all the SSc patients studied, low EBC pH or FeNO values were related to worse PFS during 4 years of follow-up. In patients with ILD, high eCO levels were associated with lower FVC at baseline and at 4 years of follow-up, although this was not shown to be prognostic.
In our study, only 1 patient from the control group developed ILD at 45 months of follow-up, showing high baseline EBC pH and FeNO levels. This shows that EBC biomarkers are not suitable for long-term prediction of SSc-ILD, but unfortunately we have no short-term data to verify these results.
Several studies have previously shown that EBC pH values may fall in non-controlled asthma, in respiratory infections in patients with bronchiectasis, COPD or cystic fibrosis.24 However, in interstitial lung diseases, higher values have been found in pulmonary fibrosis,25 and values are diminished in asbestosis.26 There are no studies in scleroderma as a marker of disease or prognosis. We found lower levels of EBC pH in patients with ground-glass opacities in HRCT, supporting the hypothesis that EBC biomarkers could be influenced by the type of inflammation present in the lungs. The prognostic role of EBC pH diminished when only non-immunosuppressed patients were studied, which can be explained by the exclusion of the most severe ILD patients and the smaller population analyzed. Inflammatory processes trigger a range of mechanisms which produce acidification of proximal airways as a possible innate defense mechanism.27 These mechanisms are the production and excretion of superoxide ions and protons by the respiratory epithelial cells, the inhibition of glutaminase activity in epithelial cells, and finally the recruitment of macrophages and neutrophils which lyse, thereby increase the acidity of the environment.28 Although the presence of lymphocytic inflammation in the alveoli is characteristic in scleroderma, neutrophilic inflammation has been shown in the bronchi, which is most marked in patients with pulmonary fibrosis.13 In fact, the acidification produced by neutrophil recruitment may well explain this inflammatory component.
With regard to IL-6, a previous transversal study negatively correlated this cytokine measured in EBC with TLC, DLCO and KCO at the moment of ILD assessment.9 In BAL, increased levels of IL-6 in SSc-ILD have been found.29 Recently, high IL-6 serum levels have been related to spirometry decline or death within the first year in patients with milder ILD disease.5 Similarly, we reported a trend towards association between high baseline IL-6 levels and lower DLCO at the end of follow-up.
In this study, using FeNO at a flow rate of 50mL/s, low baseline NO levels were correlated with baseline FVC and with a decline in both FVC and DLCO at 4 years of follow up. This study is the first to demonstrate the prognostic role of FeNO in SSc-ILD, where baseline levels lower than 10.75ppb were correlated with worse PFS. FeNO represents the quantity of NO exhaled as a mixture of the alveolar concentration of exhaled NO (CaNO) and NO diffused from the airway wall (JawNO).30 At flow rates greater than 50mL/s, the alveolar concentration of NO to the total fraction predominates, but slower rates represent airway diffusion.31 Moodley et al. found higher NO levels in SSc patients with no pulmonary fibrosis compared to patients with SSc-ILD or controls.32 Malerba et al. also showed lower FeNO levels in SSc-ILD than in patients without ILD.33 Others authors compared FeNO in SSc patients with no statistical differences between patients with or without ILD, even compared with healthy controls.34 Although difference were not statistically significant, our study and all previous studies have described lower FeNO levels in SSc patients with ILD than in patients without this organ injury.34,35
However, reports focused on analyzing CaNO have observed that this parameter is increased in SS patients compared with healthy control subjects,36 and is also particularly increased in SSc patients with ILD.12,35 High CaNO levels have been associated with decline of lung function or death, with a specificity of 90%.12
In our study, the selected flow rate may have been affected due to a low representation of alveolar space. Additionally, CaNO was not measured, which inhibits the exploration of divergent results compared with FeNO. Reduced FeNO levels may be explained by restricted NO production by NO synthase in proximal airways, as a consequence of an inhibition due to high CaNO levels secondary to inflammation of the lungs, with high alveolar generation by inducible NO synthase. Nevertheless, our data demonstrate that SSc patients with lower levels of FeNO present a worse outcome during follow-up, which may indicate the presence of an aggressive ILD. Because FeNO and CaNO represent different breath components, further studies would be necessary to elucidate the pathophysiologic role of NO in SSc-related ILD.
A novel result reported in this study is the correlation between higher levels of eCO and lower baseline FVC and at 4 years of follow-up in SS-ILD patients, although this finding has not been shown to be a prognostic factor. It is well known that many inflammatory lung diseases course with high eCO levels.37 To our knowledge, no studies have been conducted on scleroderma, although some authors have supported the possibility that pathologies with microcirculatory alterations could increase endogenous levels of CO.13,38 The fact that only 1 of the ILD patients was a current smoker and was instructed to avoid smoking enabled us to rule out tobacco as the factor responsible for this relationship.
The main limitation of the study was the small sample size, which prevented us from performing a multivariate analysis. The duration of pulmonary involvement was not homogeneous, which may have underestimated some inflammatory biomarkers due to the inclusion of patients in later stages of disease with lower lung inflammation status. Seven patients were taking immunosuppressants, which may have affected the baseline biomarkers levels. However, in the substudy excluding those patients, no substantial differences were observed, except that EBC pH was not found to have a prognostic value. The lack of measurement standardization in those biomarkers may be a limitation, as well as the non-repeatability of EBC data in studies including SSc-related ILD patients. Finally, FeNO and CaNO levels could not be correlated due to lack of CaNO data in SSc patients.
ILD has become one of the major causes of mortality in SSc, and this is why every effort must be made to find prognostic markers for this disease. This study is the first to establish the prognostic role of EBC pH, FeNO, and to analyze eCO in SSc-related ILD. Therefore, our data should be confirmed in further studies in order to clarify the correlation between FeNO and CaNO levels in scleroderma patients. In this regard, biomarkers in EB or EBC could be relevant for the management of SSc-ILD with the aid of non-invasive tests. This will lead to the identification of patients most at risk of deterioration who should be closely monitored.
FundingA. Guillen-Del Castillo is a researcher supported by the Contratos Predoctorales de Formación en Investigación (PFIS) grant from Instituto de Salud Carlos III [FI14/00643]. M.J. Cruz is a researcher supported by the Miguel Servet programme from Instituto de Salud Carlos III [CP12/03101].
Conflict of InterestThe authors declare no conflict of interest directly or indirectly related to the manuscript contents.
Please cite this article as: Guillen-del Castillo A, Sánchez-Vidaurre S, Simeón-Aznar CP, Cruz MJ, Fonollosa-Pla V, Muñoz X. Valor pronóstico del pH en el condensado de aire exhalado y de la fracción exhalada de óxido nítrico en la enfermedad pulmonar intersticial asociada a esclerosis sistémica. Arch Bronconeumol. 2017;53:120–127.