The objective of this study was to evaluate tracheal reactivity induced by a biodegradable polydioxanone tracheal stent.
Materials and methodsTwenty-two rabbits were divided into 3 groups assigned to different survival times (30, 60 and 90 days post-implantation). A biodegradable stent was implanted in each animal, except for 1 from each group (negative control). Implantation was performed through a small tracheotomy under fluoroscopic control. CT and histopathological studies were scheduled at the end of survival times.
ResultsNo animal died during the procedure or follow-up. The stent had disappeared in 100% of the cases at 90 days, in 50% at 60 days, and in none at 30 days.
CT studies revealed a greater tracheal wall thickness at 30 days than at 60 and 90 days (1.60 ± 0.41 mm in the central part of the stent versus 1.11 ± 0.18 and 0.94 ± 0.11; P = .007, respectively). No granulomas were observed on histopathology. Some degree of histological changes were noted at 30 days, which had reduced at 60 and 90 days. Differences were also found in both CT and histology between animals in which the stent was present and animals in which it had degraded.
ConclusionsPolydioxanone stents produce a mild reaction that reverts with tracheal degradation. The use of these biodegradable stents in benign tracheal disease is promising.
El objetivo de este estudio es evaluar la reactividad traqueal inducida por un stent traqueal biodegradable de polidioxanona.
Material y métodosVeintidós conejos se dividieron en 3 grupos con diferentes tiempos de supervivencia (30, 60 y 90 días postimplantación). Se implantó un stent biodegradable en cada animal, excepto en uno de cada grupo (control negativo). La implantación se realizó a través de una pequeña traqueotomía y bajo control fluoroscópico. Al finalizar los tiempos de supervivencia programados se realizaron estudios de TC y anatomopatológicos.
ResultadosNingún animal murió durante el procedimiento ni en el seguimiento. El stent había desaparecido en el 100% de los casos a los 90 días, en el 50% a los 60 días y en ninguno a los 30 días.
En los estudios de TC se observó un grosor de la pared traqueal mayor a los 30 que a los 60 y 90 días (1,60 ± 0,41 mm en la parte central del stent frente a 1,11 ± 0,18 y 0,94 ± 0,11; p = 0,007). En el estudio anatomopatológico no se encontraron granulomas. A los 30 días se observaba cierto grado de alteración histológica, la cual se reduce a los 60 y 90 días. También se encuentran las diferencias, tanto en las TC como en la histología, entre animales con el stent presente y animales con el stent degradado.
ConclusionesLos stents de polidioxanona producen una leve reacción traqueal que revierte con la degradación. El uso de estos stents biodegradables en la patología traqueal benigna es prometedor.
Benign tracheobronchial stenosis (BTBS) decreases the caliber of the trachea or the major bronchi causing airflow disturbances, and always results in severe respiratory comorbidity. It can be congenital or acquired. The most common acquired form is caused by endotracheal intubation, and there may also be other etiologies, such as inflammation, infection, trauma, and iatrogenesis.1 The conventional treatment is surgery, an approach currently considered by many surgeons as the therapy of choice.2,3 In recent years endoscopic treatments, such as balloon tracheoplasty or bronchoplasty, laser, cryotherapy, electrocautery, photodynamic therapy and local drug treatments (corticosteroids, 5-fluorouracil and mitomycin C)3–6 have been developed, but outcomes have been controversial. The alternative to surgery has been the placement of differently constructed and shaped stents.
Silicone stents,7 widely used to date, present problems with migration and accumulation of mucus causing bacterial overgrowth.8 They also require rigid bronchoscopy with general anesthesia for implantation.8,9 Self-expanding metal stents (SEMS) immediately improve symptoms8,10–12 and have significant short-term advantages, including easy implantation by flexible endoscope or under fluoroscopic guidance, obviating the need for general anesthesia, and a high stent-to-vessel ratio.8,9,12 However, these stents are associated with significant long-term complications and their withdrawal is always complicated and dangerous.8,10,13,14 Because of these complications, the U.S. Food and Drug Administration (FDA) warned against their use in benign disease.15
In order to overcome some of the disadvantages of uncovered SEMS, polymer-covered SEMS (cSEMS) have been developed. These devices have the advantages of SEMS, in that they can be extracted, but they also cause problems with superinfection.14,16,17
Biodegradable stents are beginning to be used in all anatomical territories on the grounds that they act as long as they are needed and subsequently disappear. These stents are made of biodegradable corrosive metals or polymers,18,19 polydioxanone being one of the most widely used polymers due to its long degradation time.20
The aim of this article was to study tracheal reactivity to a biodegradable polydioxanone stent (BPS) in healthy experimental animals, followed up clinically with imaging and histopathological studies.
Materials and methodsThis was an experimental study carried out with the approval of the Ethics Committee of Animal Experimentation of the University of Zaragoza, according to the ARRIVE initiative. Animals were handled and used in accordance with the Spanish Animal Protection Policy RD 53/2013, which complies with the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
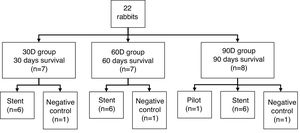
Study subjectsThe study included 22 healthy adult female New Zealand rabbits (Oryctolagus cuniculus) weighing 4.61 ± 0.52 kg. The animals were fed ad libitum with standard kibble and housed in individual cages with a grating floor intended for the species, with a light/dark cycle of 12 h.
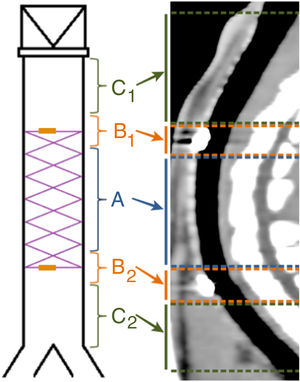
They were randomly distributed into 3 groups with different survival times (Fig. 1). All the animals in each group were implanted with a BPS, except 1 from each group which acted as a negative control.
StentThe study device was a BPS manufactured by ELLA-CS sro, Czech Republic, constructed with a single 3.5 EP polydioxanone woven filament. The stents are self-expandable, measure 8 ⋅ 30 mm and are cylindrical in shape with a radiopaque gold marker at each end. They are supplied separately from the delivery system, which had to be loaded immediately before implantation. Polydioxanone is a biodegradable polymer used since the 1980s in sutures and other prostheses. It causes little tissue reaction and is neither antigenic nor pyrogenic. This material is a polyester that is degraded by hydrolysis (accelerated in acid medium), resulting in degradation products that are eliminated in the urine or metabolized in the citric acid cycle. In suture form, the absorption time is 182–238 days.21,22
ProcedureThe animals underwent a clinical examination prior to the procedure. The implantation was carried out under general anesthesia with intramuscular administration of a combination of medetomidine (Sedator®, Eurovet Animal Health, Netherlands) (0.5 mg/kg) and ketamine (Imalgene 1000®, Merial, Spain) (25 mg/kg). The animals were kept oxygenated with a laryngeal mask and anesthesia was maintained with intramuscular injections of ketamine (0.5 mg/kg) every 20 min. Monitoring continued throughout the procedure.
In order to maintain the laryngeal mask to control the airway and oxygenate and ventilate the animals throughout the procedure, implantations were performed through a mini tracheostomy, under fluoroscopic control. In each intervention the animal was positioned in a supine posture with the neck hyperextended. A small incision was made in the neck and the tissues were dissected until the trachea could be seen. The trachea was punctured immediately caudal to the cricoid ring between two tracheal rings with a peripheral 18 G plastic trocar (Introcan®, B. Braun, Germany). A total of 0.15 ml of 5% lidocaine was administered through the plastic sheath of the trocar (B. Braun, Barcelona, Spain). A 0.035'' Teflon guidewire (StarterTM Guidewire, Boston Scientific, Ireland) impregnated with lidocaine was advanced under fluoroscopic guidance. The access was dilated with a 12 Fr dilator (12 F dilator, Cook Medical, min). The delivery system loaded with the stent (11.8 Fr) was advanced over the guidewire and the stent was released at least 1 cm cranial to the carina. Implantation was performed under fluoroscopic guidance, visualizing the radiomarkers. The same procedure was performed in the control animals, but using an empty delivery system.
Before the procedure, 50 mg/kg of oxytetracycline (Terricina LA, Pfizer, Madrid, Spain) was administered for antibiotic coverage and 0.2 mg/kg meloxicam (Metacam ®, Boehringer Ingelheim, Germany) was given for post-surgical analgesia.
Clinical follow-upTechnical success was defined as an uneventful procedure.
Following implantation, the animals were clinically monitored during the pre-defined survival period (30, 60, and 90 days depending on the group), during which physical status and the presence of respiratory symptoms were assessed. Clinical success was defined as survival to the end of the programed period and no symptoms requiring additional therapy.
Computed tomography studyAfter the scheduled survival time, CT studies (GE Healthcare Brivo™ CT325 16 slice) were performed under sedation in all animals (a CT scan was performed in the pilot animal at 30 and 90 days). The studies were performed with a slice thickness of 1 mm at 0.5 mm intervals and multiplanar reconstructions were made.
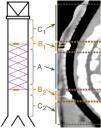
To facilitate the interpretation of the images, the trachea was divided into 5 sections, as shown in Fig. 2. If the stent had degraded, its position was estimated in relation to its location in the fluoroscopy performed during implantation.
The presence of the radiomarkers and images suggestive of granulomas were evaluated, and the thickness of the tracheal wall was measured.
Histopathological studyAfter the CT, the animals were sacrificed while still sedated with pentobarbital sodium (Dolethal®, Vétoquinol, France). Tracheas were extracted for macroscopic and microscopic study.
The samples obtained were fixed in 10% formalin, embedded in paraffin, sliced, and stained with hematoxylin-eosin. They were then divided into 3 portions (cranial, medial and caudal) for histopathological study. The degree of changes was assessed using the scoring system shown in Table 1.23
Scoring system for assessing epithelial changes in histopathological study.
| Parameter | Classification | Points |
|---|---|---|
| Epithelial thickening | No thickening (≤50 µm) | 0 |
| Mild thickening (>50−100 µm) | 1 | |
| Moderate thickening (>100−150 µm) | 2 | |
| Severe thickening (>150 µm) | 3 | |
| Unevaluable (destroyed epithelium) | – | |
| Subepithelial thickening | No thickening (≤400 µm) | 0 |
| Mild thickening (>400−600 µm) | 1 | |
| Moderate thickening (>600−800 µm) | 2 | |
| Severe thickening (>800 µm) | 3 | |
| Epithelial changes | No changes (30% caliciform cells and 70% ciliated cells) | 0 |
| Mild changes (30%–50% caliciform cells and 70–50% ciliated cells) | 1 | |
| Moderate changes (50%–70% caliciform cells and 30–50% ciliated cells) | 2 | |
| Severe changes (>70% caliciform cells and <30% ciliated cells) | 3 | |
| Epithelial destruction | 4 | |
| Squamous metaplasia | No foci of squamous metaplasia | 0 |
| Presence of squamous metaplasia foci | 1 | |
| Neovascularization | No change in quantity (<5 vessels/field) | 0 |
| Slight increase in quantity (5−15 vessels/field) | 1 | |
| Severe change in quantity (>15 vessels/field) | 2 | |
| Acute inflammation (neutrophils) | No acute inflammation | 0 |
| Mild acute inflammation (1−10 inflammatory foci) | 1 | |
| Moderate acute inflammation (>10 inflammatory or generalized foci at low concentration) | 2 | |
| Severe acute inflammation (generalized inflammation at high concentration) | 3 | |
| Chronic inflammation (lymphocytes and histiocytes) | No chronic inflammation | 0 |
| Mild chronic inflammation (1−10 inflammatory foci) | 1 | |
| Moderate chronic inflammation (>10 inflammatory or generalized foci at low concentration) | 2 | |
| Severe chronic inflammation (generalized inflammation at high concentration) | 3 | |
| Presence of granulomas | No granuloma formation | 0 |
| Presence of isolated granuloma | 1 | |
| Presence of more than one granuloma | 2 | |
| Maximum score | 21 |
All statistical data and analyses were processed using SPSS Statistics (IBM SPSS Statistics for Macintosh, version 21.0; IBM Corp., Armonk, NY, USA). An error level of 0.05 was set. Qualitative variables were expressed as frequencies and quantitative variables were described as mean ± standard deviation. Qualitative variables were compared using the likelihood ratio or Fisher's exact test. Before the comparisons, the quantitative variables were tested for normality using the Shapiro-Wilk test. If the data followed a normal distribution, Student's t test was used for independent samples (2 means) or variance analysis, ANOVA (more than 2 means). The Mann–Whitney U test or the Kruskal–Wallis test were used for non-normal distributions. The Wilcoxon test was used for paired samples.
ResultsTechnical and clinical success was 100%. During clinical follow-up, the health status of the animals was good, and no significant respiratory symptoms were observed.
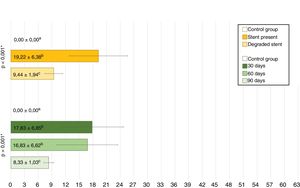
CT studyRadiomarkers were detected in all implanted animals who underwent CT at 30 days post-implantation (n = 7). They were present in half (n = 3) of the animals in the D60 group, and had disappeared in the other half (n = 3). In the D90 group, radiomarkers had disappeared in all animals (n = 7) (p < 0.001; Cramér’s V: 0.837) (Fig. 3).
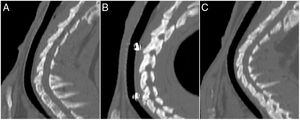
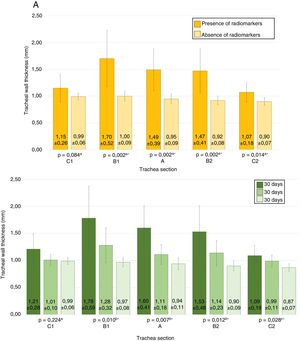
No stent migration was observed in any CT where radiomarkers were present. In CTs where radiomarkers were not observed in the trachea, they were not observed in the lung either. In 7 CTs (30.4%), images suggestive of granulomas were observed, with preserved tracheal patency in all animals. The location of the possible granulomas was: A (n = 1), B1 (n = 3) and C1 (n = 3). Significant differences were observed in area C1 (p = 0.038; Phi: 0.509) in the thickness of the tracheal wall depending on whether possible granulomas were present (1.37 ± 0.30 mm) or absent (1.02 ± 0.13 mm). No significant differences were found between controls and implanted animals in any of the studied parameters. Tracheal wall thickness results are shown in Fig. 4.
Histopathological studyIn all cases in which radiomarkers were observed on the CT, the presence of the stent was confirmed in the macroscopic study of the specimen, while in cases in which they were not, no stent remains were found in the anatomical specimen.
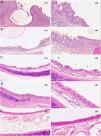
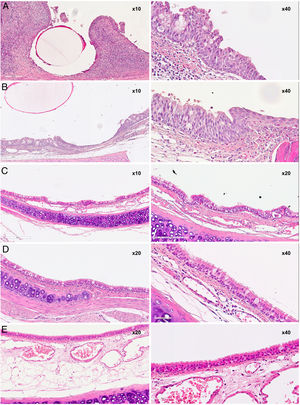
The histological study revealed no granulomas that could confirm the images observed on CT. Stent reepithelialization did not occur in any case in which the stent was still present, and only impressions of filaments in the epithelium or epithelial invaginations over the filaments that were incompletely covered were observed.
In the implanted animals, significant differences (p = 0.028) were found in the degree of histological change between area A scores (4.56 ± 2.23 points) and area C1 scores (5.22 ± 2.60 points), but no significant differences with area C2 were observed (4.56 ± 2.31 points).
The total scores are shown in Fig. 5 and the images in Fig. 6.
Overall mean scores from histopathological studies. Yellow: Scores depending on if stent was present or degraded. Green: Post-implantation time scores.
Values with the same superscript indicate the absence of statistically significant differences, according to the Mann−Whitney U test for matched values.
* Significance by Kruskal-Wallis test.
Studies performed at 30, 60, and 90 days showed histological changes in the implanted animals. In general, there was a slight increase over time in the thickness of the epithelium, and a change in the cell populations of the epithelium with an increase in the number of caliciform cells and a decrease in ciliated cells, although the cilia generally appeared normal. Both chronic and acute inflammatory foci were also observed, although in the latter the differences are not significant with respect to the control group.
In the groups sacrificed at 30 and 60 days post-implantation, a slight increase in subepithelial thickness was observed, with no significant differences between groups. In cases where the subepithelial thickness was less than 400 μm (less than that of the control animals), the connective tissue appeared denser.
The squamous metaplasia foci observed appeared as small foci surrounded by normal epithelium. The scores obtained for squamous metaplasia and neovascularization were highest in the D30 group and lower without being significantly different in the D60 group, while in the D90 group there were no foci of squamous metaplasia or neovascularization. There are significant differences between the scores of the D30 and D90 groups in both the squamous metaplasia parameter (p = 0.001) and the neovascularization parameter (p = 0.006).
With respect to the sum of the score of all the parameters, a decrease in the scores over time was observed, the mean of the scores of the D90 group being significantly different from those of the D30 group (p = 0.003) and the D60 group (p = 0.003).
Course of degradationWith respect to degradation, two situations were found: complete stent degradation and stent persistence. Significant differences in mean total scores were observed between cases where the stent was still present and those where it had already degraded (p = 0.001). The scores for subepithelial thickness, squamous metaplasia, neovascularization, and acute inflammation was 0 in all animals in which the stent had degraded, whereas in animals in which the stent was still present, the subepithelial thickness had increased in 6 of 9 animals (66.67%); the difference with the degraded group was significant (p = 0.004). Furthermore, all animals in which the stent had not degraded showed squamous metaplasia foci (p < 0.001), 8 of 9 animals showed neovascularization foci (88.89%; p < 0.001), and 7 of 9 showed acute inflammatory foci (77.78%; p = 0.002).
DiscussionThe treatment of BTBS is a real challenge. In cases in which surgery is contraindicated or otherwise ruled out, uncovered SEMS achieve rapid and significant clinical improvement.8,10–12 However, their use is associated with important long-term complications, a problem that is aggravated by difficulties associated with their removal, since they become embedded in the epithelium.8,10,13,14 In contrast, cSEMS can be removed, but problems with mucociliary transport are common.16,17 Serrano et al.24 hypothesized whether the use of paclitaxel-releasing stents would reduce the epithelial reaction and improve outcomes. However, paclitaxel-releasing stents caused significant lesions, probably due to the fact that the dose of paclitaxel should be adjusted, as drug washout in the tracheobronchial tree is much less intense than in the vascular system and there is accumulation in the epithelium. The outcomes of steel stents were also negative, and while nitinol stents caused the least reaction, their removal remains a problem.24 Recently, other authors25 have reported acceptable results with SEMS in tracheobronchial lesions.
cSEMS do not cause hyperplasia and hyperreactivity problems in the covered part, but these adverse effects are inevitable at the ends of the stent, especially in those with uncovered ends.25–27 However, the real limitations of this type of stent are problems with mucociliary changes and migration and the need for large-caliber bronchoscopes.25,27
BPS are self-expandable and adapt to the tracheobronchial wall, they do not cause any major mucociliary changes, and do not need to be removed.28–30 In the tracheobronchial tree, BPS devices have shown promising results in the treatment of BTBS30,31, lung transplants,29,32,33 tracheobronchomalacia,28,30–32,34 and external compression.28,35 BPS may play an important role in pediatric patients in whom the trachea is still developing and growing.28 Although BPS do not require a second intervention for removal, several consecutive interventions may be necessary if the degradation occurs before the remodeling of the stenosis.28,31,32,35
In our study, degradation occurred between 30 and 90 days, and was complete at 60 days in half of the animals. This is consistent with the Novotny study in which degradation was complete at 10 weeks.36 However, Stehlik et al.30 found in human patients that rapid degradation started between 85 and 94 days post-implantation. In our study, CT scans showed complete or incomplete stent degradation according to the presence or absence of radiomarkers. The question arises as to what happens to the radiomarkers: are they expelled by coughing or are they lost in the lung parenchyma? In all CT scans where radiomarkers were not in their initial position, they had disappeared completely, and were not observed in the bronchi or lung parenchyma. Nor was there evidence that stent fragments had migrated distally. These data appear to indicate that both radiomarkers and stent fragments might be expelled by coughing. In fact, in adult patients, the expulsion of stent fragments during coughing has been described; these events are not associated with complications and are well tolerated.30,32 Sztanó et al.,37 in a series of 3 pediatric patients, reported that fragments produced during degradation may behave like foreign bodies with fatal consequences. These complications in pediatric patients have not been described by other authors.28,31,34,35 The animals in our study probably did not present this problem because they were healthy adults, and more efficient in expelling the fragments.
Since BPS are self-expanding, they can be implanted using the same techniques as metal devices. However, the delivery system, which is quite rigid, has a diameter of 11.8 Fr. (3.89 mm), so a wide working channel is required. The open-cell structure of the stent should allow mucociliary transport. In our study, the epithelium appeared well preserved, and no epithelial destruction was observed, cilia were normal, and there was an increase in the proportion of caliciform cells. Foci of squamous metaplasia appeared as small areas of metaplasia surrounded by normal epithelium, which could coincide with the contact zones of the stent. The absence of granulomas in the histological study is noteworthy, since granulomas are one of the main complications of stents in both experimental and clinical studies.7,24,25,38 CT images suggestive of granulomas were not confirmed by histological results, possibly because accumulations of mucus may appear similar to granulomas on CT. Granulation phenomena have been observed in experimental and clinical studies with BPS, although they may be due to a previous lesion of the epithelium.30,39 Stehlik et al.30 believe that BPS-induced mucosal hyperplasia can help stabilize stenosis.
The results of both clinical and CT follow-up of the animals showed that tracheal patency was maintained. No cases of stent migration were recorded, despite no stent epithelialization being observed.
The main limitation of this study is that it was performed in an experimental animal model, so results cannot be fully extrapolated to humans, despite similarities between the rabbit trachea and the human trachea. Moreover, the tracheal reaction of a healthy trachea was evaluated, as opposed to a pathological trachea, which would be the case if the stent was used in a real patient. As the radial strength of the stent is unknown, we also do not know whether it can withstand stenosis.
In conclusion, this study shows that the BPS generates changes that do not impact significantly on the tracheal epithelium, which reverts to a healthy epithelium as the stent biodegrades. These results suggest that the use of these stents may be safe, although their effectiveness in a stenotic model should be studied.
FundingFinanced by the Government of Aragon, Spain (Reference Group B36_17R) and co-financed with Feder 2014-2020 “Building Europe from Aragon”.
Conflict of interestsThe authors state that they have no conflict of interests.
We thank ELLA-CS sro for supplying prostheses adapted to the animal model, Dr. Fernando Lostalé for his scientific advice, and the Centro Clínico Veterinario de Zaragoza S.L. for performing CTs.
Please cite this article as: Rodriguez-Zapater S, Serrano-Casorran C, Guirola JA, Lopez-Minguez S, Bonastre C, de Gregorio M. Stent traqueal biodegradable de polidioxanona. Estudio de la reactividad en conejo. Arch Bronconeumol. 2020;56:643–650.