Although the chronic presence of microorganisms in the airways of patients with stable chronic obstructive pulmonary disease (COPD) confers a poor outcome, no recommendations have been established in disease management guidelines on how to diagnose and treat these cases.
In order to guide professionals, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has prepared a document which aims to answer questions on the clinical management of COPD patients in whom microorganisms are occasionally or habitually isolated. Since the available scientific evidence is too heterogeneous to use in the creation of a clinical practice guideline, we have drawn up a document based on existing scientific literature and clinical experience, addressing the definition of different clinical situations and their diagnosis and management. The text was drawn up by consensus and approved by a large group of respiratory medicine experts with extensive clinical and scientific experience in the field, and has been endorsed by the SEPAR Scientific Committee.
A pesar de que es conocido que la presencia crónica de microorganismos en las vías aéreas de pacientes con enfermedad pulmonar obstructiva crónica (EPOC) en fase de estabilidad conlleva una evolución desfavorable, ninguna guía de manejo de la enfermedad establece pautas sobre cómo diagnosticar y tratar este tipo de casos.
Con la intención de orientar a los profesionales, desde la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) se ha elaborado un documento que pretende aportar respuestas clínicas sobre el manejo de pacientes con EPOC en los que se aíslan microorganismos de forma puntual o persistente. Dado que la heterogeneidad de las evidencias científicas disponibles no permite crear una Guía de Práctica Clínica, se ha elaborado un documento basado en la literatura científica existente y/o en la propia experiencia clínica que aborda tanto la definición de las diferentes situaciones clínicas como su diagnóstico y manejo. El texto ha sido consensuado entre un amplio número de neumólogos con gran experiencia clínica y científica en este ámbito. Este documento cuenta con el aval del Comité Científico de SEPAR.
Chronic obstructive pulmonary disease (COPD) presents with chronic bronchial inflammation that alters local defense mechanisms, meaning that potentially pathogenic microorganisms (PPMs) are isolated from respiratory sample cultures of 8%-43% of patients in a clinically stable phase.1,2 These isolates are more frequent in more severe cases, exacerbators, and patients with chronic bronchitis, bronchiectasis, and low peripheral eosinophil counts.3–6Table 1 lists the most common PPMs.
List of the most frequently isolated microorganisms in COPD patients128–131.
| PPM | Non-PPM |
|---|---|
| Haemophilus influenzaeStreptococcus pneumoniaeMoraxella catarrhalisPseudomonas aeruginosaOther non-fermenting gram-negative bacilli (Achromobacter xylosoxidans, Acinetobacter baumannii, Alcaligenes faecalis, Stenotrophomonas maltophilia, Pseudomonas spp., …)Klebsiella pneumoniaeOther Enterobacteriaceae (Escherichia coli, Klebsiella aerogenes, Enterobacter cloacae, Serratia marcescens, Proteus spp., Povidencia spp., Citrobacter spp. …)S. aureus, including MRSAPasteurella multocida | Streptococcus of the viridans group Gemella morbillorumNeisseria commensalsStaphylococcus epidermidis and other CoNSMicrococcus spp.Enterococcus spp. |
CoNS: coagulase-negative staph; MRSA: methicillin-resistant Staphylococcus aureus; non-PPM: non-potentially pathogenic microorganisms or usual flora; PPM: potentially pathogenic microorganisms.
The presence of PPMs in clinically stable patients has several consequences, such as increased bronchial neutrophil inflammation,3,7–11 increased sputum purulence,10,12 progressive FEV1 decline,8,13–15 worse quality of life,16,17 more frequent and more severe exacerbations,11,18,19 and higher mortality,20,21 probably due to low-grade infection that can contribute to the progression of COPD.22
The methodologies of the scientific evidence in this area vary widely and generate controversy around the definition, diagnosis, and management of these patients, so COPD treatment guidelines provide few recommendations in this regard.23,24 However, the presence of PPMs may have therapeutic implications, and may affect the use of certain treatments such as inhaled corticosteroids (ICS) or antibiotic therapy. In order to guide clinicians, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has prepared a consensus document based on the scant scientific literature available and the experience of experts. This document only addresses the diagnosis and management of COPD patients in whom PPMs are isolated from respiratory samples.
The methodology for the preparation of this document is detailed in Appendix B Online Supplement 1.
Module 1. DefinitionsGiven the poor sensitivity of sputum culture results and the limited microbiological monitoring that is usually carried out, it is difficult to prove the microbiological status of the COPD patient. No validated definitions are available for the presence of PPMs in the airway in these individuals, so the following definitions were agreed upon:
- •
Primary infection: The first isolation of a given PPM in a respiratory sample culture from a patient in a clinically stable disease stage.
- •
Chronic bronchial infection (CBI): Growth of the same PPM in at least 3 cultures in a period of 1 year, performed at least 1 month apart.
- •
Eradication: When the PPM causing the CBI is not isolated in at least 3 consecutive cultures in a 1-year period, performed at least 1 month apart.
- •
If a PPM is isolated again after eradication, it will be considered as another primary infection, provided the patient is not receiving chronic antibiotic therapy.
- •
For patients who do not exactly meet these definitions, the case should be classified as the closest in clinical terms.
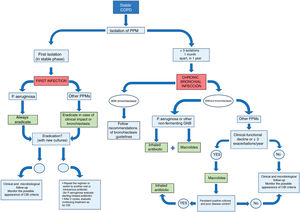
Fig. 1 shows a summary of the management of COPD patients with exceptional or persistent PPM isolates.
Module 2. Microbiological aspectsDespite their limitations, standard culture techniques are still used for the isolation of PPMs in respiratory samples,25 since molecular techniques, albeit useful in the determination and study of the pulmonary microbiota,26,27 are costly.28,29
A special case is that of Pseudomonas aeruginosa, present in 3% to 20% of stable patients with certain risk factors: FEV1 < 50%, more than 3 exacerbations in the previous year, chronic use of oral corticosteroids, bronchiectasis, admission to intensive care, and a high BODE index.5,30–33 It confers a more severe COPD phenotype than other PPMs, with more inflammation,34 exacerbations, and mortality.19,35–37
In the case of other microorganisms, isolation of Aspergillus is common in patients with certain risk factors, and is associated with more symptoms.38,39 Detection rates of non-tuberculous mycobacteria have increased in the past 10 years40: these microorganisms are associated with more frequent COPD exacerbations and accelerated functional decline.41
Agreement was reached on the following recommendations for the microbiological diagnosis and follow-up of COPD patients:
- •
Perform sputum culture as part of the initial study in high-risk COPD23 and/or in the case of persistent mucopurulent expectoration.
- •
Samples with > 25 leukocytes and < 25 epithelial cells per field (Murray-Washington grades 4−5) are valid.42 In case of non-valid samples, sampling should be repeated (especially if there is high suspicion of CBI or if P. aeruginosa has been isolated).
- •
Perform microbiological follow-up on all patients with previous PPM isolated in a clinically stable phase or with 1 of the criteria listed in Table 2.18,43–45
Table 2.Clinical criteria for deciding on the treatment of primary PPM infection other than P. aeruginosa in clinically stable patients with COPD (at least one must be met)a.
Persistent mucopurulent/purulent (Murray scale 3−8) or hemoptoic expectoration Poor disease control132,133: increased dyspnea, increased need for rescue medication, decreased physical activity, increased sputum purulence, CAT score decline Progressive worsening of lung function Frequent infectious exacerbations (≥ 2 exacerbations requiring oral antibiotic treatment or ≥ 1 requiring hospitalization or intravenous antibiotic treatment) CBI: chronic bronchial infection; COPD: chronic obstructive pulmonary disease; PPM: potentially pathogenic microorganisms.
- •
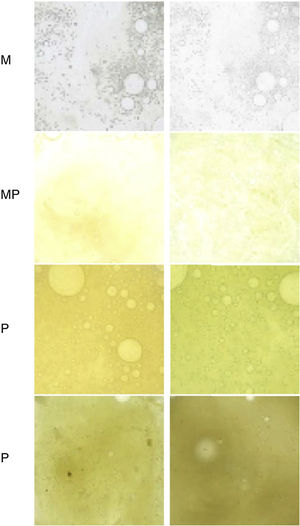
Monitor sputum color, as this is associated with the presence of PPM in stable COPD46,47 (Fig. 2).
Fig. 2.Murray scale to assess sputum color from lowest to highest purulence. M: mucoid; MP: mucopurulent; P: purulent.
Source: Reproduced with permission from Murray et al.47 - •
Cultures for fungi and/or non-tuberculous mycobacteria should be performed at least once a year, even if the patient is stable. This should also be performed in patients with: ≥ 2 exacerbations requiring systemic steroids and/or antibiotics in the last year; treatment with high-dose ICS; bronchiectasis; if radiological images are compatible with mycobacterial infection; before and during chronic macrolide treatment (for mycobacteria); and if there is no clinical improvement despite proper treatment of isolated PPMs.38–41,48–50
- •
In cases where a microorganism considered not potentially pathogenic (“mixed oropharyngeal flora” or “normal flora”) is isolated, no further action is necessary, unless there is a high suspicion of CBI.
- •
Time between collection, transport and processing of the samples must be less than 6 h. In any case, samples should not remain more than 24 h at room temperature, and should preferably be stored at 4°C rather than −20°C.51
CBI may contribute to the appearance and/or progression of bronchiectasis in COPD patients.52 Bronchiectasis is associated with more severe and symptomatic forms of COPD: increased sputum production and purulence, more comorbidities, greater dyspnea, greater bronchial obstruction, more frequent and severe exacerbations, increased bacterial burden, increased risk of CBI (particularly due to P aeruginosa) and increased risk of mortality.44,45,53–59 Therefore, detection of bronchiectasis is important, since the patient may benefit from specific treatments.60 In these patients, antibiotic treatment is essential, because while eradication is virtually impossible, it reduces bacterial counts, decreases exacerbations, and improves lung function.61 There is no evidence that the presence of bronchiectasis increases the likelihood of isolating microorganisms that are resistant to standard antibiotics.62
Agreement was reached on the following statements on the relationship between COPD, CBI, and bronchiectasis:
- •
A high-resolution computed tomography scan of the chest should be performed to assess the presence of bronchiectasis in patients with certain clinical characteristics (Table 3).51
Table 3.Clinical criteria that warrant a high-resolution computed tomography scan of the chest in patients with COPD to assess the presence of bronchiectasis.
High-risk COPD23 Frequent infectious exacerbations (≥ 2 exacerbations requiring oral antibiotic treatment or ≥ 1 requiring hospitalization or intravenous antibiotic treatment) Persistent mucopurulent or purulent expectoration Hemoptoic expectoration X-ray changes suggestive of bronchiectasis Repeated isolation of PPM (or a single isolation of P. aeruginosa) Progressive functional decline Presence of comorbidities associated with the development of bronchiectasis51 PPM: Potentially pathogenic microorganisms.
- •
If COPD and bronchiectasis co-exist in the patient, follow the definitions and therapeutic regimens proposed by the bronchiectasis guidelines with regard to the isolation of PPMs in cultures of respiratory samples.51
- •
These patients should be managed according to the treatment guidelines for both COPD23,24 and bronchiectasis.63–65
In COPD patients without bronchiectasis, there is no evidence to indicate the best eradication strategy or whether primary infection should be treated. However, P. aeruginosa, particularly mucoid strains,66 is liable to persist and, like Haemophilus influenzae, it has a tendency to form biofilms that hinder the action of antimicrobials and promote the persistence of the microorganism.67–70
Agreement was reached on the following recommendations for the treatment of primary infection (Fig. 1):
- •
In primary infection with P. aeruginosa, eradication treatment is always advisable.
- •
For other PPMs, consider eradication therapy in stable patients with bronchiectasis or at least 1 of the criteria listed in Table 2.
- •
Table 4 shows the most common PPM eradication treatment regimens.
- •
It is advisable to verify eradication with sputum cultures at least 15 days after the end of treatment. If eradication is not achieved after 2 cycles, consider treating as CBI (Table 4).
Table 4.Recommendations for antibiotic treatment in COPD patients without bronchiectasis, with PPM isolated from respiratory samples.
P. aeruginosa H. influenzae S. aureus MRSA Non-fermenting GNB other than P. aeruginosa Treatment of primary infection First option: Ciprofloxacin 750 mg/12 h p.o.Alternative: Levofloxacin 500 mg/12 h p.o. or 750 mg/24 h p.o.Duration 2−3 weeks (based on clinical improvement and tolerance) First option: Amoxicillin/clavulanic acid 875/125 mg/8 p.o.Alternatives:Amoxicillin 1−2 g/8 h p.o.Ciprofloxacin 750 mg/12 h p.o.Azithromycin 500 mg/24 h p.o.Cefditoren 400 mg/12 h p.o.Duration 10−14 days except azithromycin (6 days) and cefditoren (10 days) First option: Cloxacillin 500−1,000 mg/6 h p.o.Alternatives: Amoxicillin/clavulanic acid 875 mg/8 h p.o..Cotrimoxazole 160/800 mg/12 h p.o.Duration 2 weeks First option: Linezolid 600 mg/12 h p.o.Alternatives:Cotrimoxazole 160/800 mg/12 h p.o.Clindamycin 300−450 mg/ 6−8 h p.o.Duration 2 weeks S. maltophilia:First option: Cotrimoxazole 800/160 mg/12 h p.o.Alternative: Levofloxacin 500 mg/12 h p.o.A. baumani Imipenem 0.5−1 g/6−8 h i.v.Duration 2 weeks If not eradicated after a first treatment cycle Repeat the regimenAssess i.v. treatmentAssess inhaled antibiotic therapy Repeat the regimen or switch to another antibiotic (p.o. or i.v.) Repeat the regimen or switch to another antibiotic (p.o. or i.v.) Repeat the regimen or switch to another antibiotic (p.o. or i.v.) S. maltophilia: Minocycline 200 mg loading dose, 100 mg/12 h p.o. or i.v. Colistin: 2−3 MU/8 h or 4.5 MU/12 h i.v.A. baumani Tigecycline: 100 mg loading dose, 50 mg/12 h i.v. Colistin 2−3 MU/8 h or 4.5 MU/12 h i.v. Treatment of severe exacerbation (or first isolation detected during severe exacerbation) First option: Ceftazidime 2 g/8 h p.o. + Tobramycin 5−10 mg/kg/24 h i.v.Alternatives: Imipenem 1 g/8 h i.v. orPiperacillin/tazobactam 4 g/6−8 h i.v. orAztreonam 2 g/8 h i.v. orCefepime 1−2 g/8 h i.v. orMeropenem 2 g/8 h i.v. orCiprofloxacin 400 mg/12 h i.v. orCeftolozane/tazobactam 1−2 g/8 h i.v. or Ceftazidime-avibactam 3 g/8 h i.v. + Amikacin 15−20 mg/kg/24 h i.v. orGentamicin 5−7 mg/kg/24 h i.v.Duration 14−21 days (based on clinical improvement) First option: Amoxicillin/clavulanic acid 1−2 g/8 h i.v.Alternative: Ceftriaxone 2 g/24 h i.v.Duration 10−14 days (start antibiotic treatment i.v. and switch to p.o. when permitted by the patient’s clinical situation) First option: Cloxacillin 1−2 g/4−6 h i.v.Alternatives: Amoxicillin/clavulanic acid 1−2 g/8 h i.v.Vancomycin (dose adjusted for weight and renal function)Duration 2 weeks First option: Linezolid 600 mg/12 h i.v.Alternatives: Vancomycin (dose adjusted for weight and renal function)Ceftaroline 600 mg/8 h i.v.Ceftobiprole medocaril 500 mg/8 h i.v.Duration 2 weeks S. maltophilia:First option: Cotrimoxazole 800/160 mg/12 h i.v.Alternative: Levofloxacin 500 mg/12 h i.v.A. baumani Imipenem 0.5−1 g/6−8 h i.v.Duration 2 weeks Treatment of CBI Start inhaled antibiotic therapy with (in alphabetical order): Aztreonam lysine (inhalation solution) Gentamycin (i.v. formulation administered by inhaled route) Sodium cholistimethate (dry powder or inhalation solution) Tobramycin (dry powder or inhalation solution)Combine with long-term macrolides 1. Long-term macrolide treatment2. If not effective, start long-term (or cyclic) oral antibiotic treatment, according to susceptibility testing3. If not effective, start inhaled antibiotics with gentamicin (80 mg, twice a day, continuous treatment) or any of the specific inhalation antibiotics used in CBI caused by P. aeruginosa 1. Long-term macrolide treatment2. If not effective, start long-term (or cyclic) oral antibiotic treatment, according to susceptibility testing3. If not effective, start inhaled antibiotics with gentamicin (80 mg, twice a day, continuous treatment) or any of the specific inhalation antibiotics used in CBI caused by P. aeruginosa 1. Long-term macrolide treatment2. If not effective, start long-term (or cyclic) oral antibiotic treatment, according to susceptibility testing3. If not effective, start vancomycin inhaled antibiotic therapy (IV formulation administered by inhaled route), continuous treatment, 250 mg, twice a day Start inhaled antibiotic therapy with sodium cholistimethate (dry powder or inhalation solution)Combine with long-term macrolides CBI: Chronic bronchial infection; IV: intravenous; v.o.: oral route; MRSA: methicillin-resistant S. aureus.
The use of inhaled antibiotic (IA) therapy has increased remarkably due to its good results in the treatment of CBI in cystic fibrosis71 and bronchiectasis.72 IAs achieve high concentrations in the bronchial tree and produce few systemic adverse effects.73,74 There are no published clinical trials in COPD, but some small studies with colistin, tobramycin, and amoxicillin-clavulanic acid have reported good outcomes and few adverse effects.75–79
Agreement was reached on the following recommendations for IA treatment in patients with COPD and CBI (Fig. 1 and Table 4). In all cases, it should be confirmed that the patient is receiving correct treatment for COPD (including correct prescription, inhalation technique, and adherence).
- •
If COPD and bronchiectasis co-exist, follow bronchiectasis guidelines on the treatment of CBI with IA.63
- •
Prescribe IAs in patients with CBI caused by P. aeruginosa or other particularly virulent non-fermenting gram-negative bacilli (Table 1).
- •
Treat patients with CBI caused by other PPMs, with clinical and functional deterioration or frequent infectious exacerbations, with long-term macrolides.23,80 If positive cultures and poor disease control persist, start IA.
- •
The decision to prescribe a specific IA depends on the PPMs rather than on the susceptibility testing results, since IAs reach far higher levels in bronchial mucosa than the mean inhibitory concentration. An IA to which the PPM family is known to be susceptible must be selected.
- •
The dosage is the same as that used in bronchiectasis (Table 5).
Table 5.Antibiotics specifically designed for inhalation available on the marketa.
Dosis, regimen Administration time Inhalation system Aztreonam lysine, inhalation solution 75 mg, 3 times daily, on/offb 2−3 min E-Flow® nebulizer system (Altera) Colistimethate, dry powder for inhalation 1,662,500 IU, twice daily, continuous treatment 1−2 min Turbospin® Colistimethate, solution for inhalation 1−2 million IU, twice daily, continuous treatment Variable, depending on the nebulizer E-Flow® nebulizer system, Pari LC plus® 0.5−1 million IU, twice daily, continuous treatment 3−6 min I-neb AAD® Tobramycin, dry powder for inhalation 112 mg, 2 times daily, on/offb ∼ 6 min T-326 inhaler Tobramycin, solution for inhalation 300 mg/5 mL, twice a day on/offb Variable, depending on the nebulizer E-Flow® nebulizer system, Pari LC plus® 300 mg/4 mL, twice a day on/offb Variable, depending on the nebulizer aIn exceptional cases, depending on the type of potentially pathogenic microorganism or its susceptibility to antibiotics, parenteral formulations of some antibiotics may be administered by the nebulized route: gentamycin (80 mg/12 h continuously), vancomycin (250 mg/12 h continuously) or ceftazidime (1 g/12 h continuously).
- •
Maintain IA treatment as long as a clinical benefit is observed, i.e., the least purulent sputum possible according to the sputum color scale in Fig. 2 and reduction of exacerbations. Assess withdrawal after 6 months in case of clinical stability and negative cultures. In this case, continue with close microbiological monitoring, and if CBI reappears, give long-term treatment.
- •
Given the possible risk of allergy, bronchospasm, dyspnea, cough or hemoptysis,81–83 take certain precautions when using IAs, especially in patients with more severe COPD:
- •
- none-
Education on the use and maintenance of devices.
- none-
Pre-inhalation of fast-acting bronchodilators.
- none-
Administer the first dose in the hospital setting, either in a day hospital (with observation for 2−3 hours) or during a short hospital stay (in the most severe patients).
- none-
In severe COPD, consider the risk of IA-induced bronchoconstriction. Assess the possibility of performing spirometry before and after the first dose (reduction of FEV1 ≥ 15%).84 If bronchoconstriction is observed, consider changing the type of IA, the diluent or the volume of nebulization.
- •
Assess the risk of nephrotoxicity and ototoxicity due to aminoglycosides: avoid their use in severe chronic renal insufficiency; perform 6-monthly analytical testing during the first year (then annual); evaluate hearing loss during treatment.85
- •
If the patient performs bronchial drainage techniques or receives nebulized hypertonic saline therapy, these should precede IA.
- •
Macrolides (the most widely studied is azithromycin) modulate neutrophilic bronchial inflammation, interfere with biofilm formation, reduce bacterial load, and reduce exacerbations.86–89 Although the emergence of resistance is a possible long-term risk,90,91 the benefits of this treatment are currently thought to outweigh the risks.23,24,90,92
Agreement was reached on the following recommendations for macrolide treatment in patients with COPD and CBI (Fig. 1):
- •
Start macrolide treatment in stable patients with 3 or more exacerbations/year (moderate or severe, requiring antibiotic treatment), despite the correct core treatment.
- •
In the case of CBI due to P. aeruginosa, monotherapy with macrolides is inadvisable; instead, these compounds should be combined with IA.
- •
The regimens supported by the most evidence are: azithromycin 500 mg/day, 3 days/week, or azithromycin 250 mg daily, for 1 year. Treatment will subsequently be individualized according to clinical response (reduction of exacerbations and expectoration) and the appearance of side effects.
- •
Due to the seasonal distribution of exacerbations,93 a treatment holiday may be considered during the warm months, if there has been a long period of stability without exacerbations and little mucous secretion. Consider restarting in autumn (or before, if exacerbations reappear).
- •
Electrocardiogram, liver function tests, mycobacterial culture, and hearing evaluation should be performed before starting treatment. Assess whether to repeat these tests at least annually in prolonged treatments. Appendix B Online Supplement 2 details the requirements and precautions to be taken when initiating long-term macrolide treatment.
ICS therapy reduces exacerbations and improves symptoms and quality of life in patients with advanced COPD.94 ICS in patients with COPD, frequent bacterial exacerbations, CBI, and/or low blood eosinophils are associated with adverse effects: these compounds alter the antiviral immune response,95 modify the composition of the microbiome,96 and increase the bacterial load97 and the risk of upper airway infections, pneumonia98–100 and non-tuberculous mycobacteria.101,102
Agreement was reached on the following recommendations for ICS treatment in patients with COPD and CBI:
- •
Special precautions must be taken with the use of ICS in patients with CBI63.
- •
If they are prescribed, consider using the lowest possible dose.
- •
Re-evaluate the risk/benefit ratio of ICS use in patients who do not present: eosinophilia (persistently < 100 eosinophils/mm3) or features consistent with concomitant asthma.23
Respiratory rehabilitation and physiotherapy programs are underutilized, despite showing improvement in various health outcomes, including bronchial symptoms, respiratory function, quality of life, and risk of hospital readmission.103,104 Physical activity programs should be an integral and complementary part of respiratory rehabilitation, as they improve physical fitness and promote a healthier lifestyle in COPD patients.105
Prolonged use of mucolytics in COPD may have clinical benefits in exacerbators, especially N-acetyl cysteine and carbocysteine.93,106–109
Given the increased risk of malnutrition and increased energy requirement of COPD patients, adequate nutritional assessment, diet and nutrition are essential.110–112
The use of probiotics appears to reduce the rate of upper respiratory tract infections,113 and their potential effect also seems promising in lung infections.114 They can also help prevent diarrhea and antibiotic-induced dysbiosis.115–117
Agreement was reached on the following recommendations for other treatments in patients with COPD and CBI:
- •
A respiratory rehabilitation program (including health education, respiratory physiotherapy, muscle training, and physical activity programs) should be prescribed for patients with persistent expectoration, dyspnea grade ≥ 2, or low physical activity levels.
- •
Prolonged treatment with N-acetyl cysteine or carbocysteine may be considered in COPD patients with frequent exacerbations23,24.
- •
A nutritional assessment should be made in patients with COPD and CBI, including at least: body mass index, nutritional intake, and a longitudinal evaluation of progressive weight loss.
- •
Assess, according to the physician’s criteria, the possible benefit of the use of probiotics in patients who require several antibiotic cycles per year, coinciding with each cycle, in order to avoid diarrhea and intestinal dysbiosis.
COPD exacerbations have a very varied etiology. Bacterial etiology is usually mediated by an increase in the bronchial bacterial load,118 the acquisition of new strains of a specific bacterium,119,120 or changes in the bronchial microbiome.121 However, evidence suggests that PPMs colonizing the lower airway during the stable phase are associated with PPMs isolated during exacerbations.122
Agreement was reached on the following recommendations for the management of exacerbations in patients with COPD and CBI:
- •
A sputum sample for culture should always be collected at the beginning of the exacerbation before starting antibiotic treatment.
- •
A previous CBI should guide the choice of antibiotic according to the results of the last susceptibility testing (anticipated treatment) and the antibiotic susceptibility data of the hospital (Table 4).
- •
Adjust treatment if the result of the new culture is different from the previous one or if the clinical course of the exacerbation is unfavorable.
- •
If during an exacerbation a different PPM is isolated from that which is causing the CBI, administer antibiotic treatment covering both PPMs (Table 4)9,15,121–128
- •
In general, any exacerbation of COPD should assess risk factors for P. aeruginosa being involved.
- •
After an exacerbation involving a PPM, a follow-up culture should be performed at least 15 days after the end of antibiotic treatment whenever possible.
Agreement was reached on the following recommendations for the follow-up of patients with COPD and CBI (Table 6):
- •
The initial follow-up after primary infection is determined by the need for microbiological monitoring (to assess eradication, reduction of bacterial load, or new PPMs) and clinical monitoring (reduction of symptoms and exacerbations).
- •
Schedule follow-up visits scheduled depending on COPD severity, the frequency of exacerbations, and functional progress. In severe patients (GOLD D, FEV1 < 50% and/or chronic respiratory failure), monitoring may be required at least every 3 months; in milder or more stable patients, visits may be performed every 4−6 months.
- •
During the first 2 years after the primary infection, consider monitoring the patient's microbiological status at each visit; schedule visits at longer intervals if the patient remains stable.
- •
At least 3 sputum samples/year should be obtained, and whenever an exacerbation associated with an increase in the amount or purulence of the sputum occurs, before starting antibiotic treatment.
- •
Perform at least 1 spirometry a year to detect patients with rapid decline.24 In patients who start IA, perform a spirometry every 3−6 months during the first year, and also after severe exacerbation or a change in maintenance treatment.
Information to be recorded during follow-up visits in patients with COPD and chronic bronchial infection. Additional scans recommended.
| At each visit | Yearly | Exacerbations | Other times | |
|---|---|---|---|---|
| Clinical data | ||||
| Signs and symptoms in stable phase: Dyspnea (mMRC scale) Clinical criteria for chronic bronchitis Volume (semi-quantitative marked in a graduated vessel); color (Murray scale47) Symptoms suggestive of asthma/bronchial hyperreactivity Hemoptoic expectoration Systemic symptoms (fever, weight loss, etc.) SatO2 | ||||
| Exacerbations: Number of exacerbations with antibiotics and/or corticosteroids Number of admissions for exacerbation or home intravenous antibiotic treatment | ||||
| Impact of the disease: Quality of life: CAT questionnaire134 Severity: BODEx score135 | ||||
| Treatments | ||||
| Pharmacological treatment Smoking habit Exercise and physical activity Physiotherapy Influenza and pneumococcal vaccination | Seasonal influenza vaccinationPneumococcal vaccination (preferably 13-valent conjugate) once in lifetime | |||
| Pharmacological treatment Compliance Adverse effects Inhalation technique Satisfaction with inhalation devices | ||||
| Laboratory / microbiology | ||||
| Sputum culture | Also request culture of fungi and/or mycobacteria once a year, or suspected on clinical/radiological examination, and before/during long-term macrolide treatment (see Module 2) | |||
| Clinical laboratory testinga | At diagnosis of CBIAt the start of chronic treatment with macrolides or inhaled antibiotics; in the case of inhaled aminoglycosides, perform every 6 months | |||
| Respiratory function tests | ||||
| Forced spirometry | ||||
| 6-minute walk test | In case of clinical, functional and/or deterioration of SatO2 | |||
| Plethysmography/diffusion | According to clinical symptoms and availability | |||
| Imaging tests | ||||
| Chest X-ray | ||||
| High-resolution computed tomography | At diagnosis of CBI (to assess possible associated bronchiectasis)Every 2 years in case of rapid clinical-functional deterioration, frequent hemoptysis or risk factors for poor progressionFor all other patients every 4−5 years63 | |||
| Other complementary examinations | ||||
| Electrocardiogram | At diagnosis of CBI; before starting long-term azithromycin treatment | |||
| Evaluate hearing ± audiometry | Before starting and during long-term azithromycin treatmentPerform audiometry in case of hearing loss during treatment |
CAT: COPD assessment test; CBI: chronic bronchial infection; mMRC: modified Medical Research Council.
Assessment of inflammatory markers (C-reactive protein), alfa-1-antitrypsin,136 eosinophilia, nutritional parameters (albumin) or adverse effects of treatment (renal, hepatic function, etc.).
This document aims to provide clinicians with guidelines on how to detect, define, and treat COPD patients in whom PPMs are frequently or infrequently isolated. Given the shortage of publications on the subject, we decided to prepare a set of clinical recommendations on which consensus was reached among a broad group of experts, based on the scant literature and their abundant accumulated experience. Table 7 summarizes the statements contained in all 10 modules, all of which have achieved a broad degree of consensus. This set of recommendations will be continuously reviewed and its content will be updated as new scientific evidence emerges.
Summary of clinical recommendations for the management of COPD patients in whom potentially pathogenic microorganisms are isolated. The degree of consensus reached by the Scientific Committee is specified for each recommendation (% of reviewers who have scored each score from 1 to 5 on the Likert scale).
| Disagree(score 1 or 2) | Indifferent(score 3) | Agree(score 4 or 5) | |
|---|---|---|---|
| Module 1. Definitions | |||
| Primary infection: The first isolation of a given PPM in a respiratory sample culture from a patient in a clinically stable disease stage | 2.9 | 2.9 | 94.2 |
| Chronic bronchial infection (CBI): Growth of the same PPM in at least 3 cultures in a period of 1 year, performed at least 1 month apart | ↓ | 2.9 | 97.1 |
| Eradication: When the PPM causing the CBI is not isolated in at least 3 consecutive cultures in a 1-year period, performed at least 1 month apart | 2.9 | ↓ | 97.1 |
| If a PPM is isolated again after eradication, it will be considered as another primary infection, provided the patient is not receiving chronic antibiotic treatment | ↓ | 2.9 | 97.1 |
| For patients who do not exactly meet these definitions, the case should be classified as the closest in clinical terms | ↓ | 8.6 | 91.4 |
| Module 2. Microbiological aspects | |||
| Perform sputum culture as part of the initial study in high-risk COPD and/or in the case of persistent mucopurulent expectoration | ↓ | ↓ | 100 |
| Samples with > 25 leukocytes and < 25 epithelial cells per field (Murray-Washington grades 4−5) are valid. In case of non-valid samples, sampling should be repeated (especially if there is high suspicion of CBI or if P. aeruginosa has been isolated | ↓ | 5.8 | 94.2 |
| Perform microbiological follow-up on all patients with previous PPM isolated in a clinically stable phase or with 1 of the criteria listed in Table 2 | ↓ | ↓ | 100 |
| Monitor sputum color, as this is associated with the presence of PPM in stable COPD (Fig. 2) | ↓ | 2.9 | 97.1 |
| Cultures for fungi and/or non-tuberculous mycobacteria should be performed at least once a year, even if the patient is stable. This should also be performed in patients with: ≥ 2 exacerbations requiring systemic steroids and/or antibiotics in the last year; treatment with high-dose ICS; bronchiectasis; if radiological images are compatible with mycobacterial infection; before and during chronic macrolide treatment (for mycobacteria); and if there is no clinical improvement despite proper treatment of isolated PPMs | ↓ | 2.9 | 97.1 |
| In cases where a microorganism considered not potentially pathogenic (“mixed oropharyngeal flora” or “normal flora”) is isolated, no further action is necessary, unless there is a high suspicion of CBI | ↓ | 2.9 | 97.1 |
| Time between collection, transport and processing of the samples must be less than 6 h. In any case, samples should not remain more than 24 h at room temperature, and should preferably be stored at 4 °C rather than 20― °C. | ↓ | 2.9 | 97.1 |
| Module 3. Relationship between COPD, chronic bronchial infection, and the presence of bronchiectasis | |||
| A high-resolution computed tomography scan of the chest should be performed to assess the presence of bronchiectasis in patients with certain clinical characteristics (Table 3) | ↓ | ↓ | 100 |
| If COPD and bronchiectasis co-exist in the patient, follow the definitions and therapeutic regimens proposed by the bronchiectasis guidelines with regard to the isolation of PPMs in cultures of respiratory samples | ↓ | 5.8 | 94.2 |
| These patients should be managed according to the treatment guidelines for both COPD and bronchiectasis | ↓ | 2.9 | 97.1 |
| Module 4. Treatment of primary infection | |||
| In primary infection with P. aeruginosa, eradication treatment is always advisable | ↓ | 2.9 | 97.1 |
| For other PPMs, consider eradication therapy in stable patients with bronchiectasis or at least 1 of the criteria in Table 2 | ↓ | 2.9 | 97.1 |
| Table 4 shows the most common PPM eradication treatment regimens | ↓ | ↓ | 100 |
| It is advisable to verify eradication with sputum cultures at least 15 days after the end of treatment. If eradication is not achieved after 2 cycles, consider treating as CBI (Table 4) | ↓ | ↓ | 100 |
| Module 5. Inhaled antibiotic therapy | |||
| If COPD and bronchiectasis co-exist, follow bronchiectasis guidelines on the treatment of CBI with IA | 2.9 | ↓ | 97.1 |
| Prescribe IA in patients with CBI caused by P. aeruginosa or other non-fermenting gram-negative bacilli (Table 1), given their special virulence | 2.9 | ↓ | 97.1 |
| Treat patients with CBI caused by other PPMs, with clinical and functional deterioration or frequent infectious exacerbations, with long-term macrolides. If positive cultures and poor disease control persist, start IA | 2.9 | 2.9 | 94.2 |
| The decision to administer a specific AI or another depends not on the susceptibility testing, but on the PPM. An IA to which the PPM family is known to be susceptible must be selected | 2.9 | ↓ | 97.1 |
| The dosage is the same as that used in bronchiectasis (Table 5) | ↓ | ↓ | 100 |
| Maintain treatment as long as a clinical benefit is observed, i.e., the least purulent sputum possible according to the sputum color scale in Fig. 2 and reduction of exacerbations. Assess withdrawal after 6 months in case of clinical stability and negative cultures. In this case, close microbiological monitoring will continue, and if CBI reappears, give long-term treatment | ↓ | 2.9 | 97.1 |
| Given the possible risk of allergy, bronchospasm, dyspnea, cough, or hemoptysis, take certain precautions when administering IA: education on the use and maintenance of devices; preinhalation of fast-acting bronchodilators; administer the first dose in the hospital setting; in severe COPD (FEV1 < 50%), consider the risk of IA-induced bronchoconstriction, assessing the possibility of performing spirometry before and after the first dose (decline in FEV1 ≥ 15%) | ↓ | 5.8 | 94.2 |
| Assess the risk of nephrotoxicity and ototoxicity caused by aminoglycosides: avoid their use in severe chronic renal insufficiency; perform 6-monthly clinical laboratory testing during the first year (then annually); evaluate hearing loss during treatment | ↓ | ↓ | 100 |
| If the patient performs bronchial drainage techniques or receives nebulized hypertonic saline therapy, these treatments should precede IA | ↓ | ↓ | 100 |
| Module 6. Long-term macrolide treatment | |||
| Start macrolide treatment in stable patients with 3 or more exacerbations/year (moderate or severe, requiring antibiotic treatment), despite the correct core treatment | 2.9 | 5.8 | 91.3 |
| In the case of CBI due to P. aeruginosa, monotherapy with macrolides is inadvisable; instead, these compounds should be combined with IA | 5.8 | 8.5 | 85.7 |
| The regimens supported by the most evidence are: azithromycin 500 mg/day, 3 days/week, or azithromycin 250 mg daily, for 1 year. Treatment will subsequently be individualized according to clinical response (reduction of exacerbations and expectoration) and the appearance of side effects | ↓ | ↓ | 100 |
| Due to the seasonal distribution of exacerbations, a treatment holiday may be considered during the warm months, if there has been a long period of stability without exacerbations and little mucous secretion. Consider restarting in autumn (or before, if exacerbations reappear). | 2.9 | 11.4 | 85.7 |
| Electrocardiogram, liver function tests, mycobacterial culture, and hearing evaluation should be performed before starting treatment. Assess whether to repeat these tests at least annually in prolonged treatments | ↓ | ↓ | 100 |
| Module 7. Inhaled corticosteroid therapy | |||
| Special precautions must be taken with the use of ICS in patients with CBI | ↓ | 5.8 | 94.2 |
| If they are prescribed, consider using the lowest possible dose | 2.9 | ↓ | 97.1 |
| Re-evaluate the risk/benefit ratio of ICS use in patients who do not present: eosinophilia (persistently < 100 eosinophils/mm3) or features consistent with concomitant asthma | 5.8 | 2.9 | 91.3 |
| Module 8. Other maintenance treatments | |||
| A respiratory rehabilitation program (including health education, respiratory physiotherapy, muscle training, and physical activity programs) should be prescribed for patients with persistent expectoration, dyspnea grade ≥ 2, or low physical activity levels | ↓ | 2.9 | 97.1 |
| Prolonged treatment with N-acetyl cysteine or carbocysteine may be considered in COPD patients with frequent exacerbations | ↓ | 11.4 | 88.6 |
| A nutritional assessment should be made in patients with COPD and CBI, including at least: body mass index, calorie intake, and a longitudinal evaluation of progressive weight loss | ↓ | ↓ | 100 |
| Assess, according to the physician’s criteria, the possible benefit of the use of probiotics in patients who require several antibiotic cycles per year, coinciding with each cycle, in order to avoid diarrhea and intestinal dysbiosis | ↓ | 25.7 | 74.3 |
| Module 9. Management of exacerbations in patients with COPD and chronic bronchial infection | |||
| A sputum sample for culture should always be collected at the beginning of the exacerbation before starting antibiotic treatment | ↓ | 2.9 | 97.1 |
| A previous CBI should guide the choice of antibiotic according to the results of the last susceptibility testing (anticipated treatment) and the antibiotic susceptibility data of the hospital (Table 4) | ↓ | ↓ | 100 |
| Adjust treatment if the result of the new culture is different from the previous one or if the clinical course of the exacerbation is unfavorable | ↓ | ↓ | 100 |
| If during an exacerbation a different PPM is isolated from that which is causing the CBI, administer antibiotic treatment covering both PPMs (Table 4) | 2.9 | ↓ | 97.1 |
| In general, any exacerbation of COPD should assess risk factors for P. aeruginosa being involved | ↓ | ↓ | 100 |
| After an exacerbation involving a PPM, a follow-up culture should be performed at least 15 days after the end of antibiotic treatment whenever possible | 2.9 | 8.5 | 88.6 |
| Module 10. Follow-up of patients with COPD and chronic bronchial infection | |||
| The initial follow-up after primary infection is determined by the need for microbiological monitoring (to assess eradication, reduction of bacterial load, or new PPMs) and clinical monitoring (reduction of symptoms and exacerbations) | ↓ | ↓ | 100 |
| Schedule follow-up visits scheduled depending on COPD severity, the frequency of exacerbations, and functional progress. In severe patients (GOLD D, FEV1 < 50% and/or chronic respiratory failure), monitoring may be required at least every 3 months; in milder or more stable patients, visits may be performed every 4−6 months | ↓ | ↓ | 100 |
| During the first 2 years after the primary infection, consider monitoring the patient's microbiological status at each visit; schedule visits at longer intervals if the patient remains stable | 5.8 | 11.4 | 82.8 |
| At least 3 sputum samples/year should be obtained, and whenever an exacerbation associated with an increase in the amount or purulence of the sputum occurs, before starting antibiotic treatment | 5.8 | 14.2 | 80 |
| Perform at least 1 spirometry a year to detect patients with rapid decline. In patients who start IA, perform a spirometry every 3−6 months during the first year, and also after severe exacerbation or a change in maintenance | 5.8 | 8.5 | 85.7 |
IA: inhaled antibiotic; ICS: Inhaled corticosteroids; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 s; CBI: chronic bronchial infection; PPM: potentially pathogenic microorganisms.
This study has not received specific grants from public sector agencies, the commercial sector, or non-profit organizations.
Authors' contribution to the studyConception and design: DDRC, JLLC, MAMG.
Data acquisition: all authors.
Draft manuscript and critical review of intellectual content: all authors.
Final approval of the version submitted: all authors.
Conflict of interestsThe authors declare that they have no conflict of interests directly or indirectly related with the contents of this manuscript.
ANNEX 1 steering committee for the consensus document on the diagnosis and treatment of chronic bronchial infection in chronic obstructive pulmonary diseaseFrancisco Javier Callejas (Hospital Universitario de Albacete); Ángela Cervera Juan (Hospital General de Valencia); Marta Palop Cervera (Hospital de Sagunto); Antonia Fuster Gomila (Hospital Son Llàtzer); Alicia Marín Tapia (Hospital Germans Trias i Pujol); Xavier Pomares Amigo (Hospital Parc Taulí); Mirian Torres González (Hospital San Pedro de Alcántara); Jacinto Hernández Borge (Hospital de Badajoz); Gerardo Pérez Chica (Hospital Ciudad de Jaén); Rocío Jimeno Galván (Hospital Punta de Europa); Rafael Golpe Gómez (Hospital Lucus Augusti); Pedro J. Marcos Rodríguez (Hospital A Coruña); Pilar Cebollero Rivas (Complejo Hospitalario de Navarra); Eva Tabernero Huguet (Hospital de Cruces); Carlos Álvarez Martínez (Hospital 12 de Octubre); Concha Prados Sánchez (Hospital La Paz); José Javier Martínez Garcerán (Hospital Santa Lucía); Carlos Peñalver Mellado (Hospital Virgen de la Arrixaca); Marta García Clemente (Hospital Central de Asturias); Juan Rodríguez López (Hospital del Oriente de Asturias Francisco Grande Covián); Juan Marco Figueira Gonçalves (Hospital Nuestra Señora de la Candelaria); Guillermo José Pérez Mendoza (Hospital Dr. Negrín); Jesús Hernández Hernández (Hospital Nuestra Señora de Sonsoles); Carlos Amado Diago (Hospital Marqués de Valdecilla); Laura Pérez Giménez (Hospital Royo Vilanova); Virginia Moya Álvarez (Hospital Clínico Lozano Blesa); Alexandre Palou Rotger (Hospital Son Espases); Rosa Girón Moreno (Hospital La Princesa); Marina Blanco Aparicio (Hospital A Coruña); Annie Navarro Rolón (Hospital Mútua de Terrassa); Oriol Sibila (Hospital Clínic de Barcelona); Marc Miravitlles Fernández (Hospital Vall d’Hebron); Juan José Soler Cataluña (Hospital Arnau de Vilanova); José Alberto Fernández Villar (Hospital Alvaro Cunqueiro); Germán Peces-Barba Romero (Hospital Fundación Jiménez Díaz).
Please cite this article as: de la Rosa Carrillo D, López-Campos JL, Navarrete BA, Rubio MC, Moreno RC, García-Rivero JL, et al. Documento de consenso sobre el diagnóstico y tratamiento de la infección bronquial crónica en la enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2020;59:651–664.