To determine the frequency of obstructive sleep apnoea (OSA) and metabolic syndrome (MS) in normal weight patients and their characteristics, and to compare these with overweight and obese patients.
MethodsWe studied all patients with suspected OSA referred to the sleep laboratory from January to December 2009. OSA was diagnosed when the apnoea-hypopnoea index (AHI) was >5 and symptoms were present. MS was diagnosed according to International Diabetes Federation (IDF) criteria. The patients were distributed into 3 groups according to body mass index (BMI): normal weight (<25kg/m2), overweight (25–29.9kg/m2) and obese (≥30kg/m2).
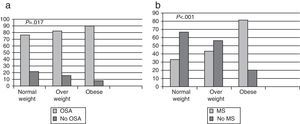
ResultsWe studied 475 patients: 7.60% normal weight and 56.4% obese. Most patients in the normal weight group were women, snorers, non-smokers, non-drinkers and were significantly younger and with a smaller neck and waist circumference than obese and overweight patients. OSA was diagnosed in 90.10%: 77.70% normal weight. OSA in these patients was mostly mild, and there were differences between the diagnosis of OSA and the BMI classified. MS was diagnosed in 64.40%: 33.33% normal weight. There was a higher probability of MS as the BMI increased. OSA and MS frequency in normal weight patients was 22% and in obese patients was 70.52%. OSA in normal weight patients was related with gender and age. There was no relationship between OSA and MS, or between otorhinolaryngological malformations and OSA in normal weight patients. Eight normal weight patients with OSA were treated with continuous positive airway pressure (CPAP) therapy.
ConclusionsThe frequency of OSA in normal weight patients was lower than in overweight and obese patients. The frequency of concomitant OSA and MS was lower in normal weight patients than in obese subjects. Normal weight patients were mostly women, younger and had no toxic habits. In normal weight patients, age and gender were predictive factors for OSA, but OSA and MS were not related.
Conocer la frecuencia del síndrome de apnea-hipopnea del sueño (SAHS)y del síndrome metabólico (SM) en normopeso y sus características. Determinar si existen diferencias epidemiológicas con aquellos con sobrepeso u obesidad.
MétodosSe estudiaron todos los pacientes con sospecha de SAHS remitidos al laboratorio del sueño desde enero a diciembre 2009. Se diagnosticó de SAHS cuando el índice de apnea-hipopnea (IAH) era >5 y existía clínica. Se diagnosticó el SM según los criterios de la International Diabetes Federation (IDF). Los pacientes se distribuyeron en 3 grupos según el índice de masa corporal (IMC): normopeso (<25kg/m2), sobrepeso (25-29,9kg/m2) y obesidad (≥30kg/m2).
ResultadosSe estudiaron 475pacientes: 7,60% normopeso y 56,40% obesos. De los normopeso, la mayoría eran mujeres, roncadores, no fumadores, no consumían alcohol y eran significativamente más jóvenes y con menor perímetro de cuello y abdomen. Se diagnosticó de SAHS al 90,10%: normopeso 77,70%. En pacientes con SAHS y normopeso la mayoría eran SAHS leve, existiendo diferencias entre diagnóstico de SAHS e IMC categorizado. Se diagnosticó de SM al 64,40%: 33,33% normopeso, encontrando mayor probabilidad de SM al aumentar el IMC. La prevalencia de SAHS y SM simultáneamente en normopeso fue del 22% y en obesos del 70,52%. El SAHS en normopeso se relacionó con el sexo y la edad. No se encontró relación entre SM y SAHS, y tampoco entre malformación otorrinolaringológica y SAHS. Se trató con CPAP a 8pacientes normopeso con SAHS.
ConclusionesLa frecuencia de SAHS en normopeso era menor que en los sobrepeso y obesos. La frecuencia de SAHS y SM simultáneamente en normopeso frente a obesos fue menor. Los pacientes normopeso eran con más frecuencia mujeres, más jóvenes y sin hábitos tóxicos. Los factores predictores de SAHS en normopeso eran sexo y edad, sin que existiera relación entre SM y SAHS.
Obstructive sleep apnoea (OSA) is a very common disease in the general population that can cause deterioration in the quality of life, hypertension, cardiovascular diseases, cerebrovascular diseases, road traffic accidents and excessive mortality in itself.1 Between 3% and 6% of the Spanish population suffer from symptomatic OSA and 24%–26% have an apnoea-hypopnoea index (AHI)>5.2 The condition is characterised by repeated episodes of upper airway obstruction, accompanied by nocturnal oxygen desaturation, fragmented sleep and excessive daytime sleepiness.1
The risk factors most associated with OSA are age, male gender, and high body mass index (BMI).2,3 Its prevalence increases with age, and is triple in elderly subjects compared to middle-aged individuals. The male/female ratio in middle age is 2–3/1, with a tendency to even out after the menopause.2 Other variables that influence the onset or development of OSA are the consumption of alcohol, tobacco, sedatives, hypnotics and barbiturates, as well as the supine decubitus position during sleep. Genetic, family and racial factors may also be involved.1
Various studies have related OSA with metabolic syndrome (MS). MS refers to a cluster of metabolic abnormalities that are predictive of an increased risk of cardiovascular diseases and type 2 diabetes mellitus. According to various studies, there have been modifications in the definition of this syndrome, with MS currently being considered as a multimorbid condition in which the fundamental components are obesity, insulin resistance, hypertension, hypertriglyceridaemia and low high-density lipoprotein cholesterol (HDLC). The exact prevalence of MS is unknown and varies substantially between various countries and according to the criteria used (National Education Program. Adult Treatment Panel III [NCEP ATP III], World Health Organisation [WHO], International Diabetes Federation [IDF], etc.), with figures of 27.3% in Canada, 20.95% and 23% in the San Antonio cohort according to whether WHO or ATP III criteria are used, and 13.2% and 16.55% according to the European Group for the Study of Insulin Resistance (EGIR) or the WHO, respectively, in France. In Spain, the National MS Register (MESYAS register) established a prevalence of 10% in active workers of both sexes. Despite these differences, there is one common fact, which is that it is becoming increasingly prevalent as obesity becomes more widespread.4,5
It has been shown in the literature that both entities are closely related, with obesity as a risk factor for its development and exacerbation. Based on this, our objective was to determine the prevalence of OSA and MS in thin patients, as well as their epidemiological characteristics, and to determine whether it differed from those who were overweight or obese.
Materials and MethodsRetrospective, observational study of all patients referred to the Complexo Hospitalario Universitario de Ourense (CHUO) sleep respiratory disorders unit (SRDU) outpatient department for suspected OSA, from January to December 2009. Cases were collected using the SRDU database. The variables collected were: age, sex, origin of referral, reason for consultation, profession (pensioner, active worker, regular driver, others), history of hypertension (HT), depressive syndrome, score on the Epworth scale, use of sedatives, BMI, neck circumference, waist circumference, otorhinolaryngological (ORL) malformations, smoking, alcohol, polysomnogram, respiratory polygraph, sleep parameters (AHI, number of desaturations per hour, mean oxyhaemoglobin saturation), diagnosis, MS, hyperglycaemia, low HDLC, hypertriglyceridaemia, and continuous positive airway pressure (CPAP) treatment. OSA was diagnosed by polysomnography (PSG) or respiratory polygraph (RP) when the AHI was >5, with consistent clinical symptoms. It was classified into 3 grades: mild (6–15), moderate (16–30) and severe (≥30). MS was diagnosed according to IDF criteria.6 Patients were then distributed into 3 groups according to BMI: normal weight (BMI<25kg/m2), overweight (BMI 25–29.9kg/m2) and obese (BMI≥30kg/m2).7
Statistical AnalysisStatistical analysis of the data was performed using SPSS program version 15.0. The quantitative variables were expressed as mean±standard deviation (SD) and the qualitative variables as frequencies and percentages. The normality of the variables was determined using the Kolmogorov–Smirnov test. The χ2 test was used to determine the association between qualitative variables; one-way ANOVA was used for the Gaussian quantitative variables and the non-parametric Kruskal–Wallis test for the non-Gaussian variables. To determine the relationship between the study variables and OSA in the group of normal weight patients, the χ2 (categorical variables) and non-parametric Mann–Whitney U tests (continuous variables) were used. A P value <.05 was considered statistically significant in all analyses.
ResultsDuring the study period, 486 patients attended the SRDU clinic; 9 were excluded due to absence of BMI data. Finally, 475 patients were studied, of whom 36 (7.60%) were normal weight, 171 (36%) overweight and 268 (56.40%) obese. Most of the patients were referred from primary care (278; 58.5%) and Respiratory Medicine (79; 16.6%).
Of the normal weight patients, most were women (20; 55.60%), with a mean age of 53.64±16.36 years, significantly lower than in the other groups (P=.021). They had a neck circumference of 36.33±3.52cm, smaller than in the other groups (P<.001), and a waist circumference of 90.42±13.48cm, also significantly smaller (P<.001). With respect to toxic habits, 24 (66.70%) were non-smokers, 30 (83.30%) did not drink alcohol regularly and 7 (19.40%) used sedatives. Normal weight patients were more often active workers (22; 61.10%).
With respect to the reason for consultation, the most common cause in normal weight patients was snoring (23; 63.90%). Daytime hypersomnolence measured using the Epworth scale was significantly lower (P=.006) in normal weight patients. In the sleep study parameters, a lower AHI (12.08±9.67; P<.01), lower CT90 (10.77±26.65; P<.001), fewer desaturations per hour (P<.001) and higher mean oxyhaemoglobin saturation (94.33%±2.81; P<.001) were observed in normal weight patients with respect to the other groups.
MS had a lower overall frequency in normal weight patients (33.33%; P<.001), and a lower frequency of individual MS criteria was also observed (P<.001). Hyperglycaemia and HT were the predominant MS criteria in normal weight patients (Table 1).
Clinical and Epidemiological Characteristics of Patients Stratified by BMI and P Value.
| Normal weightn=36 | Overweightn=171 | Obesen=268 | P | |
| Malesa | 16 (44.40%) | 135 (78.90%) | 202 (75.40%) | <.001 |
| Age, yearsb | 53.64±16.36 | 55.57±14.38 | 58.55±12.27 | .021 |
| BMI, kg/m2b | 23.02±1.78 | 27.63±1.39 | 35.63±5.77 | – |
| Neck circumference, cmb | 36.33±3.52 | 40.60±3.24 | 44.76± 4.03 | <.001 |
| Waist circumference, cmb | 90.42±13.48 | 100.97±8.08 | 118.97±12.13 | <.001 |
| Epworthb | 9.64±5.24 | 11.26±5.74 | 12.45±5.46 | .006 |
| Smokera | 5 (13.90%) | 21(12.6%) | 51 (19.60%) | .160 |
| Alcohola | 6 (16.70%) | 43 (25.1%) | 105 (39.20%) | .001 |
| History of HTa | 9 (25.70%) | 65 (38%) | 161 (60.10%) | <.001 |
| Sleep studya | 0.779 | |||
| Polysomnographya | 16 (44.40%) | 71 (41.50%) | 105 (39.20%) | – |
| Respiratory polygrapha | 20 (55.60%) | 100 (58.50%) | 163 (60.80%) | – |
| AHIb | 12.08±9.67 | 22.30±18.52 | 37.31±25.17 | <0.001 |
| CT90b | 10.77±26.65 | 10.56±19.31 | 28.23±31.95 | <0.001 |
| Mean oxyhaemoglobin saturationb | 94.33±2.81 | 93.68±2.60 | 91.31±3.77 | <0.001 |
| Metabolic syndrome and components | – | |||
| MSa | 11 (33.30%) | 69 (43.90%) | 208 (80.90%) | <0.001 |
| Hyperglycaemiaa | 13 (39.40%) | 64 (41.80%) | 189 (73.80%) | <0.001 |
| HTa | 11 (33.30%) | 70 (45.80%) | 182 (71.10%) | <0.001 |
| Low HDLCa | 8 (24.20%) | 69 (44.80%) | 141 (54.90%) | 0.002 |
| Hypertriglyceridaemiaa | 2 (6.10%) | 37 (24.20%) | 96 (37.50%) | <0.001 |
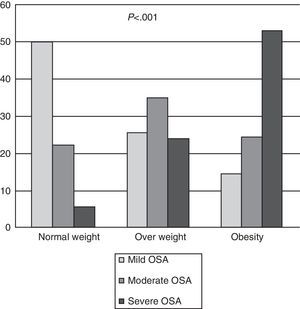
In total, OSA was diagnosed in 428 patients (90.10%); in the group of normal weight patients, the frequency was 77.70%, in the overweight group, 84.79%, and in obese patients, 91.40% (Fig. 1a). In normal weight patients with OSA, most (64.28%) had mild OSA, overweight patients had moderate OSA (41.38%) and 57.90% of obese patients had severe OSA. There were significant differences (P<.001) between the diagnosis of OSA and the BMI classified (Fig. 2). It should be noted that in the group of normal weight patients, OSA was diagnosed in 28 patients (77.70%), of whom 13 (46.40%) were female.
MS was diagnosed in 288 patients (64.40%): 33.33% in normal weight patients, 43.94% in overweight and 80.93% in obese subjects. There was a higher probability of having MS (P<.001) as the degree of obesity increased (Fig. 1b).
The frequency of concomitant OSA and MS in normal weight patients was 22%, compared to 70.52% in obese subjects (P<.001). OSA in normal weight patients was related with sex (P=.039; being female reduced the risk) and age (P=.045; patients were older). No relationship was found between MS and OSA in normal weight patients (P=.421), or between ORL malformation and OSA in this group (P=.990).
With respect to treatment, in addition to the hygiene and dietary measures recommended in all patients with OSA, 8 normal weight patients were treated with CPAP (22.20%), 71 patients in the overweight group were treated using this method (41.50%) and in the obese group, 177 (66%).
DiscussionThe frequency of OSA in normal weight patients was significantly lower than in overweight and obese subjects, as was the frequency of concomitant OSA and MS. This is consistent with findings reported in the literature, since obesity is the principal risk factor for both OSA and MS.1
In our study, normal weight patients who attended the clinic were more often women, younger, with no toxic habits and with sedative use similar to the other groups.
With respect to gender, it should be said that although more normal weight women attended the clinic, the percentage of normal weight men diagnosed with OSA was higher (15 compared to 13), although there were no significant differences.
In our study, we observed that being female reduced the risk of OSA, a finding similar to that reported in previous studies, where it has already been confirmed that being male is a risk factor for OSA in the general population. In an article by Martínez-Rivera et al.,8 it was reported that women must have a protective factor, since their study found that, despite having a higher BMI than men, they had a lower AHI. Other studies also share this theory,9–11 some of which propose female hormones as a protective factor.12
Contrary to that observed in another study,13 which found a significantly higher prevalence of the use of sedatives in non-obese patients (52.9% compared to 24.7% in obese subjects) and a higher mean age in non-obese patients (57.1 years compared to 48.3 years in obese subjects), in our study normal weight patients were younger and the use of sedatives was similar to that observed in overweight and obese patients. The younger age at OSA diagnosis and lower use of sedatives may be due to the high degree of suspicion of OSA, because of the substantial awareness that our primary care colleagues have of this condition, which means that patients are referred earlier for assessment.12 In fact, patients were most often referred from primary care, followed by Respiratory Medicine, both normal weight patients and those in the other two groups.
Normal weight patients, like the overweight patients, were more often active workers, while the obese patients were usually pensioners. The most common symptom for consulting in the 3 groups was snoring, followed by excessive daytime sleepiness and then respiratory arrests.
Ninety percent of patients referred to the clinic were diagnosed with OSA. Most normal weight patients had mild OSA, in the overweight group most were diagnosed with moderate OSA and in the obese group most were severe OSA, with significant differences between the diagnosis of OSA and the BMI classified. This implies that as the degree of obesity measured by the BMI increases, so too does the severity of the OSA, being most severe in the more obese patients. This can be observed on comparing the sleep test parameters (AHI, CT90, number of desaturations per hour, mean oxyhaemoglobin saturation), in which statistically significant differences were found between normal weight patients and the other two groups. However, in the study by Namyslowski et al.,14 which compared sleep parameters between overweight and obese patients, a significant relationship was found between the increase in BMI and sleep parameters in obese subjects only, but not in overweight patients.
Controversy remains in the literature about whether the BMI15 or waist circumference8,16,17 is the best predictor of OSA. In a previous study by our group,18 a significant relationship was found with the waist circumference but not with the BMI. With respect to the group of normal weight patients, an association was only observed with sex and age.
One condition required for the diagnosis of MS, according to IDF criteria in the European population, is the presence of central obesity, defined as a waist circumference ≥94cm in men and ≥80cm in women. In our study, only 11 of the 36 patients with a normal BMI met this criterion; these patients had truncal obesity (increased waist circumference) but not generalised obesity (BMI≤25kg/m2), and it was in these patients in whom MS was diagnosed (11/36).
The 4 possible MS criteria were not met in the normal weight patient group in any case; most met one or no criteria, with diabetes mellitus being the most common, followed by HT. However, it was observed that, in the total sample, as the degree of obesity increased, the number of criteria met also increased. Thus, in the overweight patient group, one or two criteria were met, and in the obese group, two or three; the most common criterion met in both groups was HT, followed by diabetes mellitus. The least frequently met criterion was hypertriglyceridaemia.
We know that in the general population there is a higher probability of having MS as the BMI increases, as was observed in our study.
Moreover, the relationship between MS and OSA in the general population has been demonstrated in the literature, but we did not find this relationship in our normal weight patients. In the study by Kono et al.,19 conducted in non-obese patients (BMI<30kg/m2, which included normal and overweight subjects) with and without OSA, it was suggested that even non-obese patients with OSA were susceptible to developing MS. The study by Lin et al.20 also showed that OSA was independently associated with dyslipidaemia, HT and at least 2 MS criteria in non-obese patients (BMI<25kg/m2).
It should be noted that our study cannot be compared with these, since the study by Kono et al.19 differs from ours in that we made a distinction between non-obese patients, separating them into normal weight and overweight patients, and women were also included. With respect to the study by Lin et al.,20 in this case our normal weight patients were similar to theirs (BMI<25kg/m2) and they also included women. However, their normal weight patients had a waist circumference also consistent with slimness, excluding those who had a waist circumference >90cm in men and >80cm in women. In our case, the waist circumference was not compatible with slimness in 11 patients. These individuals were those with MS, which may be influencing the result.
In the pathogenesis of OSA, ORL abnormalities have also been implicated, such as nasal blockage, amigdalar or uvula hypertrophy and soft palate, among others. The most common in our normal weight patients was mild-moderate palate hypertrophy which did not require any type of intervention. In our study, no relationship was found between ORL malformation and OSA in normal weight patients, since of the 36 normal weight patients, only 11 had some type of malformation; of these only 9 (81%) had OSA and 18 (75%) did not have any malformation but did have OSA (P=.990).
Our study has several limitations. First of all, it may be biased, since our subjects were very selected patients, referred to a specific sleep disorders study unit due to suspected OSA. This is also a retrospective study, with all the implications that that this entails. Thirdly, the number of normal weight patients was very small, which although expected given the involvement of obesity in both OSA and MS, may limit the study. Finally, the presence of menopause, which is known to influence the prevalence of OSA in women, was not recorded.
FundingThis study was conducted without any type of funding.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Dacal Quintas R, Tumbeiro Novoa M, Alves Pérez MT, Santalla Martínez ML, Acuña Fernández A, Marcos Velázquez P. Síndrome de apnea-hipopnea del sueño en pacientes normopeso: características y comparación con pacientes con sobrepeso y obesidad. Arch Bronconeumol. 2013;49:513–517.