Asthma is a public health problem that affects individuals of all ages. It is mediated by eosinophil infiltration and increased T-cell receptor (CD4+), T helper 2 (Th2) and killer T cell (TK) levels that trigger the output of inflammatory mediators.1 The role of interleukin 6 (IL-6) in promoting effector T cell subsets suggest that it may play a functional role in asthma. It has been reported that IL-6 levels in induced sputum are particularly elevated in asthmatic subjects compared with healthy controls.2 Airway inflammometry has emerged as a critical consideration in targeted therapies for the management of asthma. The aim of the present study was to evaluate whether IL-6 levels in exhaled breath condensate could differentiate between healthy and asthmatic children, and in the latter group between steroid-naïve and those receiving steroids for asthma management.
We performed a cross-sectional, comparative study on asthmatic and healthy children between 7 and 12 years of age. Children with asthma were recruited from the Pediatric Outpatient Clinic of the Regional General Hospital in Leon, Mexico. Healthy controls were recruited from a public elementary school in the same city. The study was approved by the Research Committee of the Department of Medicine and Nutrition of the University of Guanajuato (registration number 358-12).
The medical and sociodemographic history was obtained by administering the questionnaire proposed by the International Study of Asthma and Allergies in Childhood (ISAAC), validated in a previous study.3 Lung function was measured by forced spirometry. Spirometries were performed using the EasyOne spirometer (NDD, Technopark, Zurich Switzerland), which complies with the quality criteria established by the American Thoracic Society (ATS) in 1994.4 Each student underwent a forced spirometry to obtain the following parameters: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC ratio (%), and peak expiratory flow (PEF).
Exhaled breath condensate (EBC) was collected before the forced spirometry according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines,5 using an R-tube (Respiratory Research, Inc., Austin, TX). The exhaled air flowed through a condenser (precooled to −70°C), and 1.2ml (range 0.8–1.8) of breath condensate was collected during a period of 15–20min. Immediately following collection, EBC was collected and aliquots of 100μL stored in small plastic tubes at −80°C.
IL-6 levels were measured with a specific enzyme immunoassay (BioSource International, Inc., USA), at a wavelength of 410nm.
According to the ISAAC questionnaire, the frequency of respiratory symptoms (cough, wheezing, and rhinorrhea) and allergic diseases (rhinitis and eczema) was higher in asthmatic children than in healthy controls.
The percentage of predicted FEV1 and FEV1/FVC in non-asthmatic children was significantly higher than in asthmatics (P=.02 and P=.04, respectively). According to spirometric values, 15 of the 51 children in the asthmatic group (29.4%) and 7 of the 52 healthy controls (13.4%) had impaired lung function (OR=2.3, 95% CI: 1.05–5.1). As expected, “obstructive-type” impaired lung function was more common in asthmatics (OR=5.1, 95% CI: 1.1–22.1).
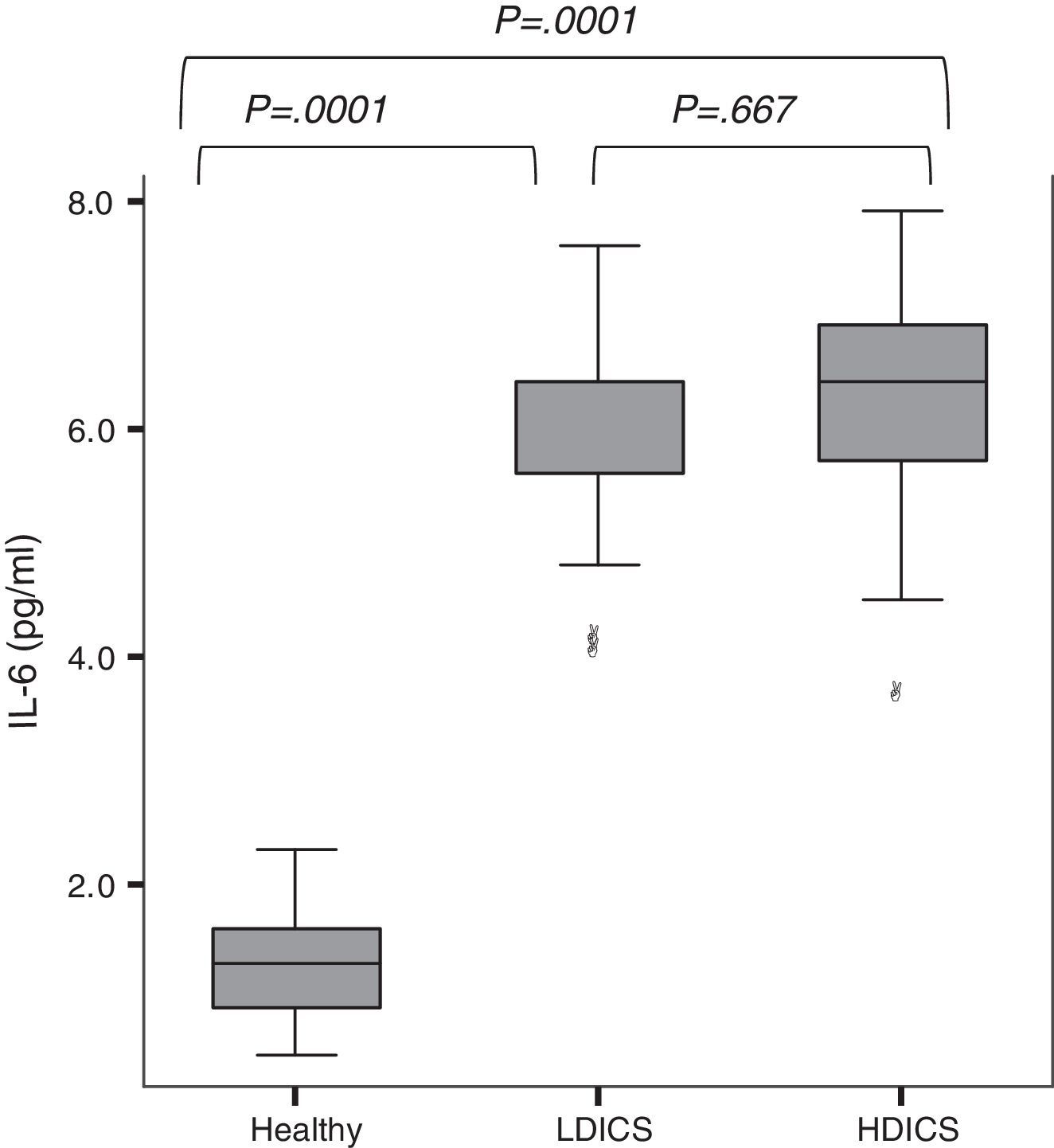
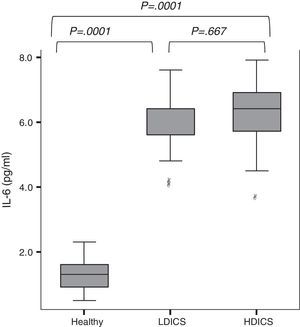
IL-6 was detectable in the exhaled breath condensate of all children, with a median value of 2.3pg/ml (95% CI: 1.7–5.3). As shown in Fig. 1, exhaled IL-6 levels were significantly higher in asthmatics than non-asthmatics (6.0±0.9 versus 1.3±0.4pg/ml; P=.0001). The analysis of variance with post hoc Tukey's test showed that IL-6 levels in healthy children were lower than in asthmatics, regardless of the steroid dose (P=.0001).
IL-6 levels in exhaled breath condensate in healthy children and asthmatics. The analysis of variance with post hoc Tukey's test showed that IL-6 levels in healthy children were significantly lower than in asthmatics, regardless of steroid therapy (P=.0001). There was no difference in levels of IL-6 among asthmatics. HDICS, high-dose inhaled corticosteroids; LDICS, low-dose inhaled corticosteroids.
In the multiple regression model, after adjusting for age, gender and anthropometric variables, factors associated with IL-6 levels were: being asthmatic (β=4.5, P=.0001), history of atopy (β=−0.5025, P=.0001) and FEV1/FVC (β=−0.013, P=.03). The area under the receiver operating characteristic (ROC) curve showed that IL-6 value ≥3.6 were able to predict asthma with a sensitivity of 93%, specificity of 88%, positive predictive value of 88% and negative predictive value of 93%.
Few studies have measured IL-6 in the exhaled breath condensate as a biomarker of lung inflammation, and of the few that have focused on asthmatic patients, most involved a small sample size. In this study, we present the results in a larger population than previously reported. A limitation of our study was our failure to conduct tests to diagnose concommitant diseases; high levels of IL-6 have been reported in endothelial cells and bronchoalveolar lavage fluid of patients with chronic diseases such as lupus, sarcoidosis, chronic obstructive pulmonary disease, and also in EBC of patients with cystic fibrosis.
Conclusion: IL-6 levels in EBC in asthmatic children were significantly higher than in healthy children. IL-6 exhaled ≥3.6pg/ml showed a sensitivity of 93% and specificity of 88% to differentiate asthmatic children from healthy children.
We would like to thank Norma Amador Licona PhD, Juan Manuel Guízar Mendoza PhD, and Estela Núñez Lemus MD for their help in interpretin the data and revising the manuscript.
This study was supported by the Public Health Institute of the State of Guanajuato, ISAPEG grants (CS-3O130108).
This article was supported in part by a grant titled: Training in Environmental Health to Reduce Chronic Disease in Latin America del Mount Sinai School of Medicine (D43 ES 018745) funded by the National Institute of Environmental Health Sciences, Dr. Luz Claudio, Principal Investigator.
Please cite this article as: Linares Segovia B, Cortés Sandoval G, del Rosario Estrada Pacheco F. Incremento de interleucina-6 (IL-6) en condensado de aire exhalado de niños asmáticos. Arch Bronconeumol. 2017;53:82–83.