Smoking is a large-scale health problem. According to the World Health Organization, 40% of children are exposed to passive smoking in the home.1
The prevalence of childhood asthma in Spain is estimated at 10%.2 Bronchial hyper-reactivity in these children makes them more vulnerable to environmental pollutants, such as tobacco smoke, which increases airway inflammation, bronchial secretion, and airflow limitation.3
A multicenter study conducted by the Working Group on Smoking in Childhood and Adolescence of the Spanish Society of Pediatric Pulmonology found a prevalence of passive smoking of 37%, which was associated with exacerbations among children with asthma.4
Few publications in our setting have objectively studied changes in lung function parameters and the severity of acute asthma exacerbations among asthmatic children exposed to passive smoking.4–7 Lung function impairment is known to be more common among infants born to mothers who smoke,8,9 and children exposed to tobacco during their development have altered lung capacities and a greater risk of developing asthma.10,11
In this study, we analyzed the prevalence of passive smoking in asthmatic children and its effect on the severity of acute episodes and lung function. This was a retrospective analysis of patients aged 4–16 years who required hospitalization for acute asthma in the Hospital General Universitario Gregorio Marañón, Madrid, Spain, from 2011 to 2015. Asthma diagnosis, severity of exacerbations, and changes in lung function, such as FEV1/FVC ratio <80% according to the GEMA 2017 guidelines, were evaluated.12 Most variables included in the statistical analysis did not show a normal distribution. The statistical tests used were the Mann–Whitney U test and Kruskal–Wallis for continuous variables, and Fisher's test or Chi-squared test for categorical variables. The study was approved by the Clinical Research Ethics Committee.
The analysis included 365 patients. Median age was 5 years (IQR 4–7) and median length of stay was 4 days (IQR 3–5); 63% of the children were boys, and 45% had a previous diagnosis of asthma. Median body mass index (BMI) was 16kg/m2. Concomitant atopy was diagnosed in 65%.
Forty-one percent (n=151) were passive smokers (6%, mother smoked; 14%, father; 19%, both; 2%, other family members).
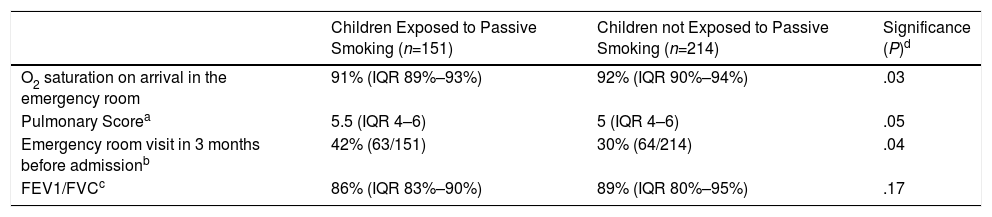
Table 1 shows the analysis of some severity markers observed in asthmatic children, according to exposure to passive smoking. Patients exposed to passive smoking had lower oxygen saturation levels on admission, higher scores on the asthma exacerbation severity scale, worse lung function parameters, and more visits to emergency departments in the previous months (P<.05).
Bivariate Analysis of Severity Parameters of the Acute Asthma Episode in Study Patients (n=365) Associated With Passive Smoking.
| Children Exposed to Passive Smoking (n=151) | Children not Exposed to Passive Smoking (n=214) | Significance (P)d | |
|---|---|---|---|
| O2 saturation on arrival in the emergency room | 91% (IQR 89%–93%) | 92% (IQR 90%–94%) | .03 |
| Pulmonary Scorea | 5.5 (IQR 4–6) | 5 (IQR 4–6) | .05 |
| Emergency room visit in 3 months before admissionb | 42% (63/151) | 30% (64/214) | .04 |
| FEV1/FVCc | 86% (IQR 83%–90%) | 89% (IQR 80%–95%) | .17 |
Pulmonary Score (PS): scale to evaluate severity of the asthma exacerbation, used in our study.12 A score of 0–3 is assigned to each of the sections analyzed (breathing, wheezing, and use of accessory muscles), providing a score of 0–9. Mild exacerbation: 0–3; moderate: 4–6; severe: 7–9.
Data expressed as a percentage of patients who attended at least 1 emergency room visit in the same hospital for any asthma-associated event in the 3 months prior to the exacerbation for which they were admitted. The remaining data in the table are expressed as median and interquartile range.
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity. Obstruction in children is defined as a FEV1/FVC ratio <80%.12
Asthma exacerbations were more severe when the father was the smoker (PS 6; IQR 4.2–6.7), compared to the mother or both parents (PS 5, IQR 4–6); P=.05. Moreover, passive smokers had significantly more previous episodes of bronchiolitis (68% vs 56%, P=.02; OR 1.5) and bronchospasm (80% vs 65%, P=.02).
Spirometry was performed in 55% of the children within 2 months following the acute episode to provide information on their baseline asthma severity,12 and lung function impairment was detected in 25% (n=51).
In the group of asthmatic children exposed to smoking, the FEV1/FVC ratio was lower if the smoker was the mother (median 86%, IRQ 83%–90%) than if the father was the smoker (89%, 85%-105%), both parents were smokers (87%, 77%–92%) or neither smoked (89%, 80%–95%), although these differences were not significant (P=.07). The proportion of cases with impaired lung function (FEV1/FVC<80%) was greater if both parents smoked (33.3%), than if only the father (7.7%) or the mother (13.3%) smoked (P=.03), irrespective of severity.
A multivariate analysis was performed to evaluate the association between independent variables such as age, sex, and passive smoking, with impaired lung function as the dependent variable. The logistic regression model showed that the absence of passive smoking was an independent protective factor against impaired lung function (P=.029; OR 0.44; 0.204–0.919). A second multiple linear regression model was used to evaluate the association between age, sex, atopy, and passive smoking, with the severity score as the dependent variable. This showed that passive smoking (β 0.2, 0.032–0.98; P=.037) and atopy (β 0.3, 0.2–0.98; P=.002) were associated with higher severity scores.
With respect to tobacco exposure in the home, while the literature has placed greater emphasis on the role of the mother,3,8 we found that asthma exacerbations were more severe when the father was the smoker (P=.05). This may be related with differential factors between parents, such as amount and type of tobacco smoked. Previous studies have shown an association between greater lung function impairment and a greater number of cigarettes smoked by fathers.6,11
Although some earlier observations found higher susceptibility to passive smoking in girls,13 no differences were found in our series.
Other potential risk factors, such as age, sex, atopy, pets, and nutritional status, also occurred in our series.12 It is important to highlight that the multivariate analysis revealed only passive smoking as an independent risk factor for impaired lung function in asthma, and both passive smoking and atopy for exacerbation severity.
The study is limited by its retrospective nature, which prevented us from collecting data on the frequency and burden of smoking by parents in the home. Nor were cotinine levels determined in urine, as this test was not routinely available in our laboratory.14
Not only is second-hand smoking a preventable risk factor,7 it is also one of the main causes of worsening of asthma5 and the main environmental determinant of lung function decline.15 In our series, 41% of the children were passive smokers, a proportion in line with previous studies, yet very high, considering these children were asthma patients.4
Knowing that the quality of life of these children may be significantly compromised,15 we believe it is essential to insist on measures to prevent passive smoking in the family environment.10
Please cite this article as: Blázquez ML, Moreno JP, Vázquez SV, Fernández RR. Impacto del tabaquismo pasivo en la función pulmonar y gravedad del asma en la población pediátrica. Arch Bronconeumol. 2018;54:436–437.