Tuberculosis still is a major public health problem worldwide, and vaccines may play a major role in its eradication. However, despite 20 years of intensive research, we still do not have a better vaccine than the Bacille Calmette-Guérin vaccine, which has been used since 1921 but exhibits only limited efficacy in the field. This effort has not, however, been entirely in vain as our understanding of TB vaccinology has been substantially expanded and there are currently 17 vaccine candidates in clinical development and several more in preclinical trials. This manuscript reviews the most important recent advances, concerns raised and future prospects in the TB vaccinology field.
La tuberculosis (TB) continúa siendo un importante problema de salud pública a nivel mundial, para cuya erradicación las vacunas pueden jugar un papel relevante. Sin embargo, a pesar de 20 años de intensa investigación, todavía no se dispone de una mejor alternativa que la vacuna del bacilo Calmette-Guérin, que se utiliza desde 1921 pero cuya efectividad es limitada en el campo. Sin embargo, este esfuerzo no ha sido en vano, porque nuestro conocimiento sobre la vacunología de la TB ha aumentado sustancialmente, haciendo que actualmente haya 17 vacunas candidatas en estudios clínicos y algunas más en fase preclínica. Este artículo revisa los avances más importantes, problemas y perspectivas de futuro en el campo de la vacunología de la TB.
Tuberculosis (TB) remains a major public health challenge worldwide, especially in those regions where the disease is most prevalent, such as South-East Asia, Africa and the Western Pacific Region.1 Despite all the efforts devoted in this regard, the goals set by the WHO to tackle TB by 2015 were not met.2 A vaccine against TB is likely to play an important role in the resulting new scenario as this is considered to be one of the major interventions needed to achieve a reduction in TB incidence of 17% per year by 2035 instead of the current 1.5%, thus hypothetically reaching an incidence of about 10 cases/100,000 people per year by 2035.3 Moreover, the growing number of cases due to multi-resistant and extremely resistant M. tuberculosis (Mtb) strains (MDR-TB and XDR-TB), co-infection with HIV, the presence of comorbidities and/or socio-economic issues complicate the pathway to TB eradication even further.1,4
The Bacille Calmette-Guérin (BCG) vaccine, an attenuated, live M. bovis-based vaccine that has been administered worldwide since 1921 with positive effects, is one of the strategies currently available to tackle TB.5,6 This vaccine, which is given to neonates, does not fully prevent pulmonary TB but decreases the risk of disseminated and severe forms of TB disease in childhood, an effect that is particularly important in highly endemic countries.6 However, its efficacy varies depending on the region in which it is administrated, possibly due to environmental pressures exerted by non-TB mycobacteria (NTM) or even altitude; and Magntani's meta-analysis showed its protection to be higher in those individuals with no prior Mtb infection than in those already latently infected, thus suggesting that BCG confers better protection against pulmonary TB if administered before school age.7,8 Although it may last for 40 years in some cases, it is well known that the protection conferred by the BCG vaccine wanes with time, and the amount of time needed for this to happen has been calculated to be approximately 10–20 years post-administration, thus coinciding with the maximum peak of TB incidence during a person's lifetime.8–10 The decrease in protection despite BCG persistence has been related to impaired functional abilities of both CD4+ and CD8+ T cell responses.11 Revaccination to overcome this problem is still a common practice in countries of the former Soviet Union,12 and several clinical studies assessing the benefit of revaccination reported different outcomes, either positive or negative, with different implications for public health policies.13–16 The question of when to revaccinate remains unclear, although it seems that this should be done once the memory T cell responses are established, rather than at the peak of the BCG response, in order to avoid exhaustion effects.11 The origin of prime-boost strategies with either BCG or other vaccine candidates relies on the concept of making the BCG effect last longer, and even improve it, while being safer. In this sense, it seems important to thoroughly study the results obtained in experimental animal models and to extract as much information as possible in order to be able to determine the best administration schedule for each candidate.
With regard to safety, administration to neonates has caused problems in countries where HIV infection is highly prevalent as immunosuppression in children makes them more vulnerable to disseminated disease involving BCG.17,18 Both the limited protection of this vaccine and the fact that it is a live (although attenuated) vaccine, with all the inherent safety concerns, explain the need to search for new vaccine candidates. A new TB vaccine should ideally perform better than BCG but also have a better safety profile. But how much better, if there still are so many things we do not know about the BCG? The scientific community is fully aware that we should study the BCG in greater depth to better understand it and use all this information to design better vaccines or improved schedules.10
A better route of administration and optimized prime-boost strategy schedule could be possible given the large amounts of data already published or gathered in the context of epidemiological studies conducted worldwide, as well as in the clinical trials and experiments carried out in animal models to evaluate new candidates in which BCG has been used as a control. All this information would also need to be combined with our understanding of the mechanism of action.10 In this sense, and in addition to revaccination schedules, researchers are currently seeking for administration routes other than intradermal that could confer an increased protection (in terms of quality and quantity) on BCG or any other candidate aiming to replace it; these studies have mostly been carried out in experimental animal models.
BCG was first administered to humans orally, although this route was rapidly superseded by other, more immunogenic routes. Over the past few years, several studies have been carried out with vaccine candidates, including lipid-based formulations to increase the immunogenicity when administered orally.19 Other approaches include the administration of BCG and other TB vaccine candidates via mucosal vaccination (aerosol, intranasal or intratracheal) as the primary route for Mtb infection is via aerosol. The literature shows that this route is more protective against Mtb infection than subcutaneous vaccination, at least in animal models.10,11,20 Even more so than when delivered mucosally, Sharpe et al.21 have shown that BCG vaccination via the intravenous route is even more effective than all other routes tested, at least in terms of immunogenicity and efficacy. Although this raises safety issues as regards scalability to human vaccination, this information could be essential for designing new TB vaccine candidates or vaccination schedules, at least in terms of understanding the impact of a vaccine on the immune responses.
In addition to their route of administration, TB vaccines can be grouped according to their nature: whole cell (alive or inactivated) or subunit vaccines (protein construct with an adjuvant or viral-vectored); when they should be given (pre-exposure (prophylactically) or post-exposure (therapeutically)); and in which regimen (prime-boost, how many boosts, etc.). So which is better? A whole cell vaccine, which is supposed to trigger a more complete immune response? Should it be alive, as is the case for BCG, so it can replicate and prolong the immune response? But if it is alive, how safe is this given the safety issues that arise after administration of BCG in immunocompromised patients?
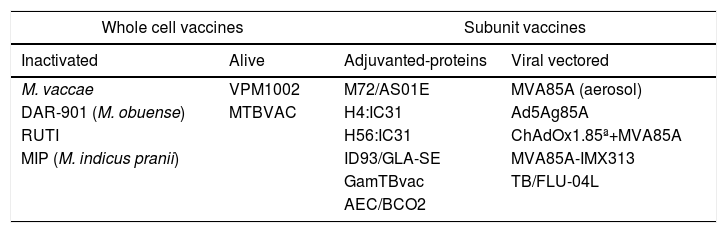
Whole cell vaccinesThere are two live vaccine candidates in the global pipeline22 intended to replace BCG while being safer (Tables 1), namely VPM1002 and MTBVAC. VPM1002 is a recombinant Mycobacterium bovis rBCGΔureC::Hly+ whose safety and immunogenicity have been demonstrated in three clinical trials (CT). This candidate is currently in phase II/III in the clinical development pathway to check its efficacy in preventing the recurrence of TB (https://clinicaltrials.govNCT03152903).11,23 MTBVAC was generated from an Mtb strain and carries two independent attenuating mutations in the transcription factor phoP and the lipid biosynthesis gene fadD26, respectively. After showing its efficacy in experimental animal models and two phase I clinical trials in adults and children,24,25 it is now being evaluated in a phase Ib/IIa study (NCT02933281).
Vaccine candidates in clinical development.
| Whole cell vaccines | Subunit vaccines | ||
|---|---|---|---|
| Inactivated | Alive | Adjuvanted-proteins | Viral vectored |
| M. vaccae | VPM1002 | M72/AS01E | MVA85A (aerosol) |
| DAR-901 (M. obuense) | MTBVAC | H4:IC31 | Ad5Ag85A |
| RUTI | H56:IC31 | ChAdOx1.85ª+MVA85A | |
| MIP (M. indicus pranii) | ID93/GLA-SE | MVA85A-IMX313 | |
| GamTBvac | TB/FLU-04L | ||
| AEC/BCO2 | |||
RUTI, DAR-901, M. vaccae and M. indicus pranii (MIP) are the four inactivated whole-cell vaccine candidates currently in the pipeline. The RUTI vaccine26 was primarily designed as a therapeutic vaccine,27 although it was subsequently found to be effective as a prophylactic in animal models.28 After a successful phase IIa trial in South Africa,29 it is now being tested as co-adjuvant treatment in MDR-TB patients (Phase IIa, NCT02711735). DAR-901 is a whole-cell vaccine manufactured in compliance with Good Manufacturing Practice from SRL172, which is derived from inactivated M. obuense (initially thought to be M. vaccae), and which was used in a successful phase III trial to show the efficacy and safety for the prevention of disseminated TB in HIV-positive patients (DARDAR study, NCT00052195).30 DAR-901 is now being tested in a phase II clinical trial as a booster to BCG for preventing infection with TB (NCT02712424). Another vaccine made from inactivated M. vaccae31 is being tested in a Prevention of Disease (PoD) phase III clinical trial in China in 10,000 Mtb-infected adults (NCT01979900) and as an adjunct to standard chemotherapy in the form of an oral pill (V7) in a phase III clinical trial in the Ukraine involving MDR-TB patients (NCT01977768). The MIP vaccine, which is used against leprosy and has already been tested as adjunct therapy in three TB clinical trials, will be soon evaluated together with VMP1002 in a phase III PoD trial in 19,000 household contacts of people with TB in India.32
Subunit vaccinesVaccines are commonly developed by generating constructs containing Mtb antigens (alone or in combination), which are delivered together with adjuvants or viral-vectored. The advantage of these candidates is that they are safer but they potentially have the drawback of being less immunogenic, as whole cell vaccines, due to their complexity, can induce conventional and non-conventional T cell and antibody responses and can train the immune system against TB as well as against other microorganisms, as has been shown for BCG.33 The primary objective of most vaccine candidates is either to increase the generation of protective lymphocyte cell subsets, to improve their presentation or to induce antibody-mediated immune responses. The triggering of an increased lymphocyte-mediated response has been the main strategy against TB during the past 20 years, although the results have shown that this may not be sufficient in terms of efficacy.34,35 In this sense, and although controversial for many years, it has recently been shown that humoral responses may play a more important role than commonly thought.36,37 The most recent research has focused on identifying new Mtb antigens using new techniques, including in silico approaches, some of which are expressed “in situ”, or lipid antigens,38 and newer and better adjuvants, as well as improved viral vectors or nanoparticles to carry antigens and thus improve their delivery to antigen-presenting cells.39
Adjuvanted subunit vaccinesThere are currently 11 vaccine candidates in the global pipeline22 that fit into this category: six of these include a protein plus an adjuvant (M72/AS01E, H4:IC31, H56:IC31, ID93/GLA-SE, GamTBvac and AEC/BCO2) and five are viral-vectored (MVA85A (aerosol), Ad5Ag85A, ChAdOx1.85ª+ MVA85A, MVA85A-IMX313 and TB/FLU-04L).
H4:IC31 and H56:IC31, both of which are in phase IIa, are the most advanced in terms of clinical development. Both are fusion proteins with the adjuvant IC31, which is a T-cell stimulator. H56:IC31 is based on ESAT-6, Ag85B and Rv2660c Mtb antigens, and is now being evaluated in two phase II clinical trials, one concerning the prevention of infection in healthy adolescents (NCT03265977) and another assessing its safety and immunogenicity when administered to MDR-TB patients in combination with standard chemotherapy and a COX-2 inhibitor (NCT02503839). H4:IC31 is based on Ag85B and TB10.4 Mtb antigens and its ability to prevent Mtb infection has recently been evaluated in a phase II clinical trial in South Africa, in which showed it to be safe and immunogenic.15 Although, in terms of the main efficacy endpoint, it didn’t prevent QuantiFeron (QTF) conversion, it nevertheless reduced the rate of sustained QTF conversion, thus suggesting some level of protection.15,32
ID93/GLA-SE is based on Rv2608, Rv3619, and Rv3620 Mtb antigens with the GLA-SE adjuvant. After a successful phase I trial,40 a phase IIa trial to evaluate the safety and immunogenicity of ID93+ GLA-SE was carried out (NCT02465216). In this clinical trial, the vaccine was administered to adult pulmonary TB patients following successful completion of TB treatment with confirmed bacteriological cure.
GamTBvac is a recombinant subunit vaccine consisting of two mycobacterial fusion proteins with an adjuvant that has just completed a phase I clinical trial in BCG-vaccinated adults (NCT03255278).41
The last adjuvanted subunit vaccine to enter clinical development is AEC/BCO2, the safety of which is currently being evaluated in a phase I clinical trial (NCT03026972).
Viral-vectored subunit vaccinesMVA85A, ChAdOx1.85ª+ MVA85A and MVA85A-IMX313 are all based on the modified Vaccinia Ankara virus expressing antigen 85A (MVA85A). MVA85A alone in infants previously vaccinated with BCG and administrated intradermally showed no increased efficacy when compared to BCG vaccination in a phase IIb clinical trial34 even though it had been found to be highly immunogenic in previous studies. As the reasons for this could be manifold, efforts to improve the immunogenicity of MVA85A are ongoing. New phase I trials are now evaluating administration via aerosol inhalation in LTBI-infected adults (NCT02532036).42 In another strategy, MVA85A has been combined with IMX313, a protein with adjuvant effect designed to boost immunity primed by BCG that was found to be well-tolerated and immunogenic in a phase I clinical trial.43 Finally, MVA85A has also been evaluated in combination with a replication-deficient chimpanzee adenovirus expressing Ag85A (ChAdOx1.85A), both in experimental animal models44 and in a phase I clinical trial (NCT01829490). Another approach involving Mtb antigen 85A involves Ad5Ag85A, a recombinant replication deficient human adenoviral (Ad5) TB vaccine containing the immunodominant antigen Ag85A that is intended to be delivered by aerosol. A phase I clinical trial is being conducted in BCG-vaccinated individuals (NCT02337270).
M72/AS01E is a subunit vaccine candidate based on two Mtb antigens (32A and 29A) with the AS01E adjuvant, which has been evaluated in two IIb trials. In a first trial assessing the safety and immunogenicity in tuberculosis patients, the vaccine triggered persistent humoral and T-cell-mediated responses but the recruitment was terminated prematurely due to a high incidence of important local adverse reactions in M72/AS01E-vaccinated individuals, raising safety concerns.45 The candidate has been recently evaluated in a phase IIb PoD CT in infected individuals (NCT01755598) showing clinically acceptable safety profile and a protection of 54% compared to placebo,46 a success which gives hope for TB vaccines for first time after the MVA85 trial Phase IIb results.
Future prospectsThe efforts of the European Community (EC), AERAS, TBVI and the Bill and Melinda Gates Foundation to boost the TB vaccine field scenario over the last 20 years have been remarkable. The resulting injection of a mixture of funds and trust, and fostering of the networking and collaboration among the key scientists in this field, has led to major advances in terms of our understanding of protective immunity, the most appropriate animal models to be used and TB vaccine research and development. Numerous candidates have been generated in the past 20 years, and the EC alone (through the framework programs FP5, FP6, FP7 and H2020) has funded TB vaccine-related research that has generated half the TB vaccine candidates in the current global pipeline.38 Moreover, significant efforts have been made to harmonize protocols, technological platforms, and a lot of know-how has been generated, including on gating, downselecting candidates and the use and refinement of experimental animal models.47–49 Despite this, more funds are constantly needed if these candidates are to be moved forward in the clinical development process and reach the market. The licensing of a vaccine requires proven efficacy in the target population and the cost of a phase III clinical trial is very high. As we have mentioned above, the experience with BCG shows that it is less effective in people who have already been exposed to Mtb, and approximately 90% of latently infected individuals will never develop TB, so this is probably because the infection itself confers a certain degree of protection.50 Moreover, it is likely that what matters most in terms of developing active disease is the infective dose, as has been shown in experimental animal models. This is supported by epidemiological findings as those at highest risk are the close contacts of TB patients, and the risk of reinfection is higher in highly endemic settings.51,52 As such, in terms of a prophylactic vaccine intended to prevent TB infection, this leaves us little room for improvement as any candidate should do better than the infection itself, which indeed is already remarkably good.50
Moreover, considering all ages, forms of disease and the different distributions of TB endemicity and prevalence of comorbidities, it is highly unlikely that a single vaccine could be used for everything.38 In this context, mathematical modeling could help to identify which populations may benefit the most from a TB vaccine and should be included in the development of future TB vaccines.53
A vaccine that is able to reduce the risk of pulmonary TB (PoD) is needed as this would be a breakthrough in terms of stopping transmission. This could be achieved using a pre- or post-exposure vaccine that can decrease the risk of progression toward active TB. However, achieving an appropriate sample size to demonstrate efficacy in these conditions would imply large and lengthy clinical trials, which represents a high cost. As no definitive correlate of protection exists, in order to reduce the size, duration and cost of such clinical trials while predicting efficacy or identifying the individuals at high risk of developing TB, clinical endpoints are needed. Focusing on specific populations can help to reduce the sample size while demonstrating the biological activity of vaccine candidates.22 An assessment of the prevention of Recurrence (PoR) during the first year post-treatment (when most recurrences occur) can act as a surrogate endpoint, although the results will need further confirmation in larger trials for a vaccine to be licensed.
Although M. vaccae, RUTI and DAR-901 have been in the pipeline for a long time, therapeutic vaccines have only come into the spotlight very recently.26,54 The aim of therapeutic vaccines is to increase the cure rate of treatment regiments and/or to reduce recurrence rates. There are well-established WHO definitions for these concepts that imply evaluation at the end of chemotherapy (6 months for DS-TB and up to 2 years for MDR/XDR-TB).55 However, as the cure rates in drug-sensitive TB cases are very high at the end of treatment, the room for improvement is limited. In MDR and XDR-TB cases, in contrast, the recruiting rate can be even longer, thereby increasing the cost and hampering the feasibility. In order to mitigate this problem, month 2 sputum conversion rate for DS-TB and month 6 for MDR/XDR-TB should be considered as endpoints in all trials as both have been considered to correlate well with a patient's final outcome.56,57 Any improvement in terms of time to stable negativization of the sputum would provide a reasonable indication for using the vaccine to shorten treatment and could have a marked impact on controlling the spread of the disease.58 However, it is important to stress that the rate of negative outcomes in MDR/XDR-TB is so high that is worthwhile to design new strategies involving a vaccine administered as coadjuvant to chemotherapy.26,59
In summary, after 20 years of intensive research we still do not have a vaccine that is able to replace BCG and there are still many unknowns in this field.35,51 However, our understanding of TB vaccinology has been substantially expanded and there are up to 17 candidates in the global pipeline, with several more yet to enter clinical development.22,49 We are confident that if funding levels are maintained, the scientific community will be able to harvest the results of all the work done over the past few years and that a brighter future lies ahead.
Conflict of interestPJC was the inventor of RUTI and one of the founders of Archivel Farma, the company that developed the vaccine, and was its CSO until 2013. CVM worked on the preclinical and clinical development of the RUTI vaccine.
CVM and PJC are supported by the European Commission Horizon 2020 research and innovation program under grant agreements No. 643381 (TBVAC2020) and 643558 (EMI-TB), and are members of the CTVD Program of the Bill and Melinda Gates Foundation. Their research groups receive funding from the Plan Nacional I+D+I co-financed by the ISCIII-Subdirección General de Evaluación and the EU Regional Development Fund (FEDER) through CP13/00174, PI16/01511, PI17/01511 and the CIBERES (CB06/06/0031). The Experimental Tuberculosis Unit is accredited by the Catalan Agency for the Management of University and Research Grants with code 2017 SGR500 and the IGTP is a member of the CERCA network of Catalan research institutes. The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.