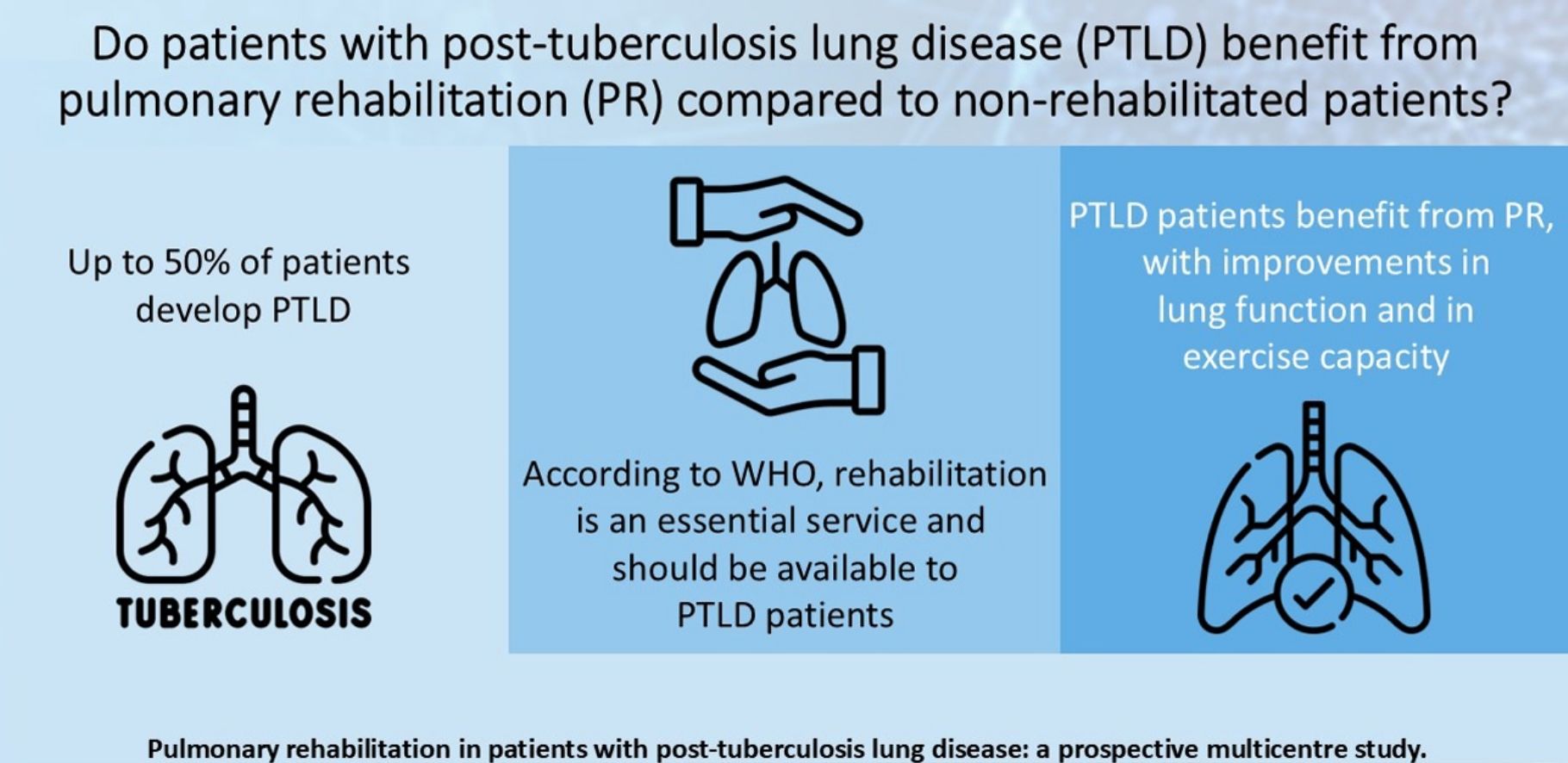
The majority of the studies on pulmonary rehabilitation (PR) in post-tuberculosis lung disease (PTLD) were from a single center, had a low sample size and/or did not allow a complete functional evaluation before and after PR program (PRP). The objective of this study was to evaluate if PTLD patients had benefits from PR in a large collaborating multicentre study conducted in reference centers in Brazil, Italy and France.
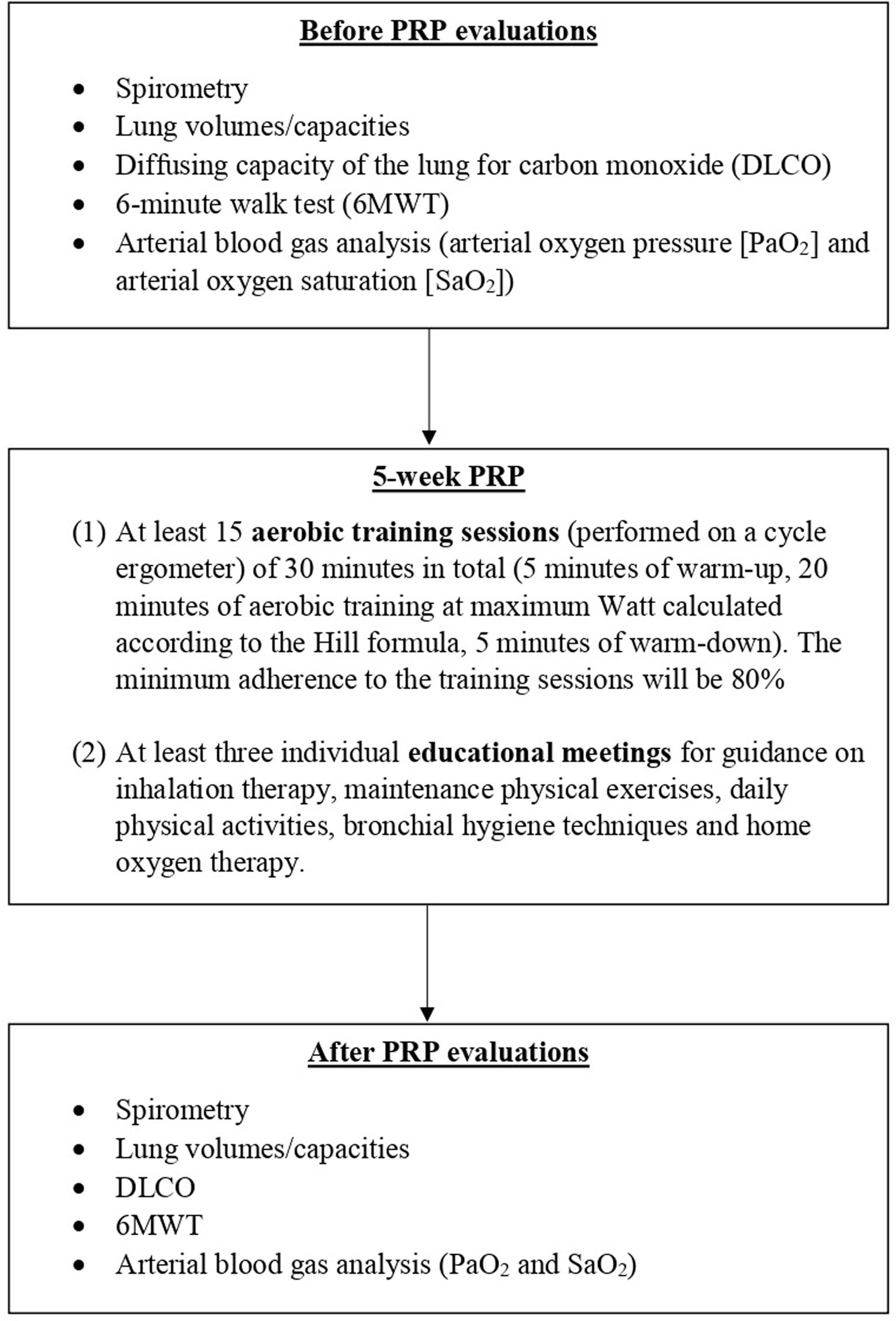
MethodsPTLD patients underwent a comprehensive 5-week PRP (Group 1), and were compared with non-rehabilitated patients (Group 2). Pulmonary function tests, 6-minute walk test (6MWT), and arterial blood gas analysis were measured two times: before and at the end of the PRP (Group 1), and at the beginning of the follow-up and after 3 months (Group 2).
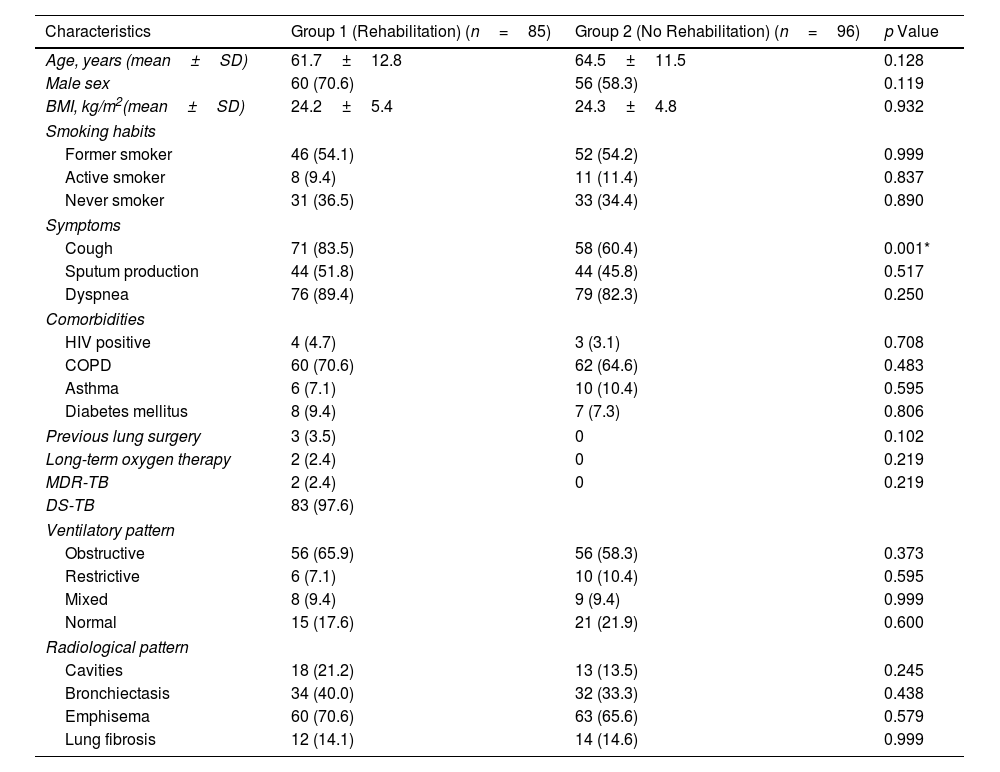
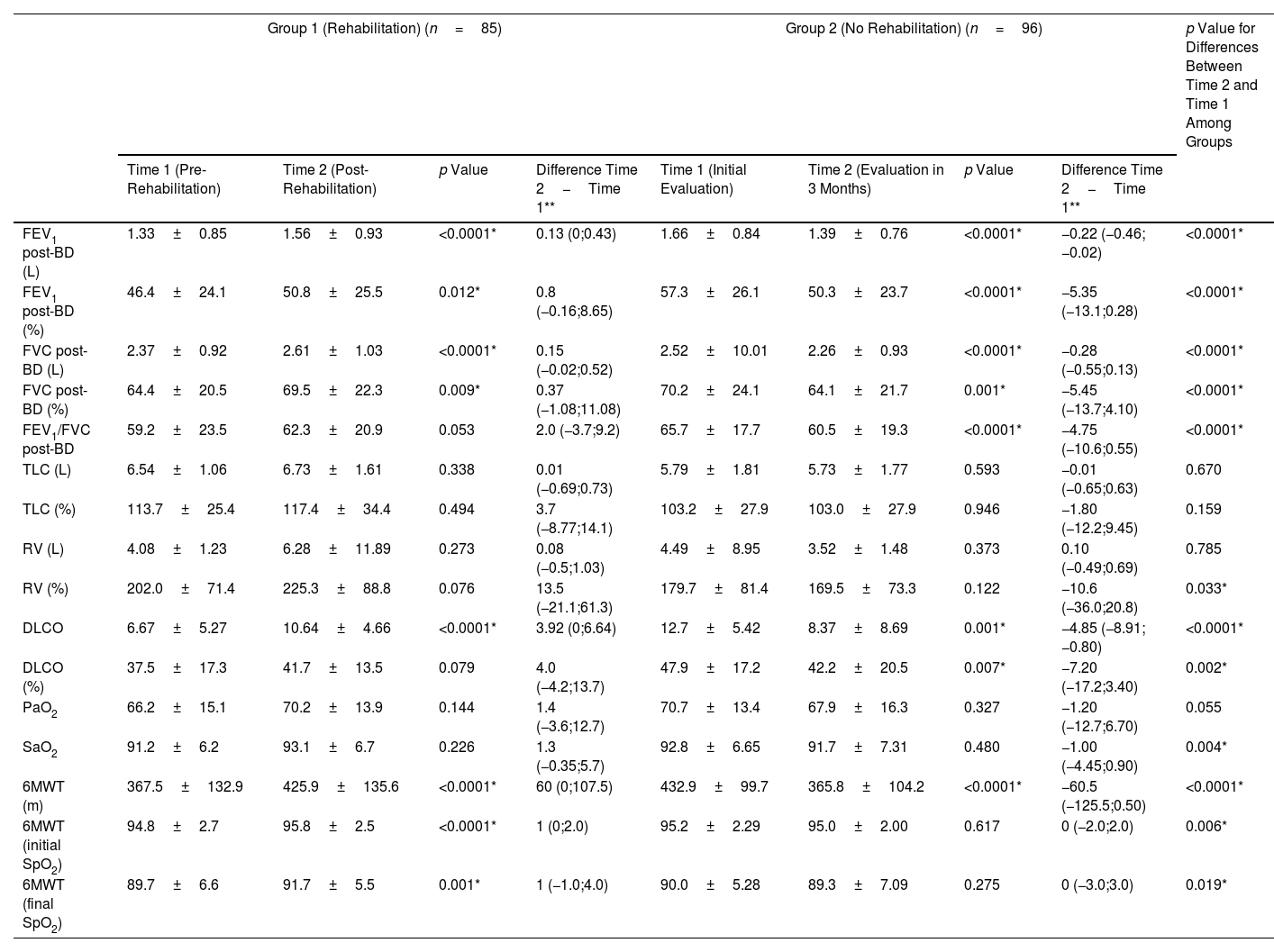
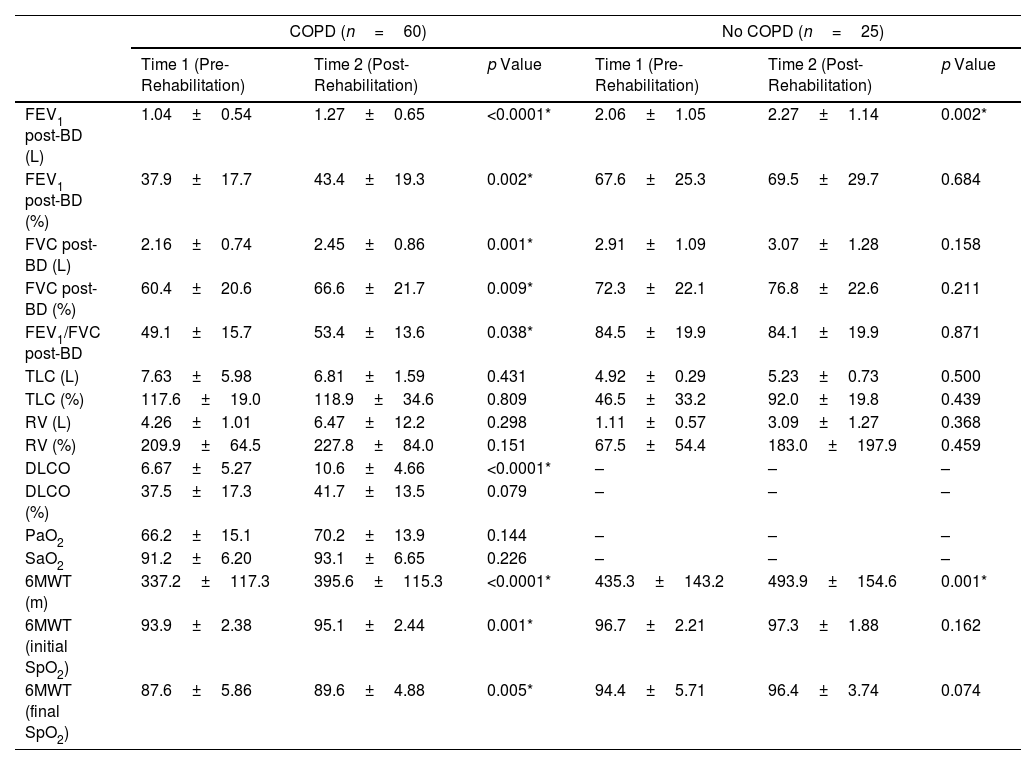
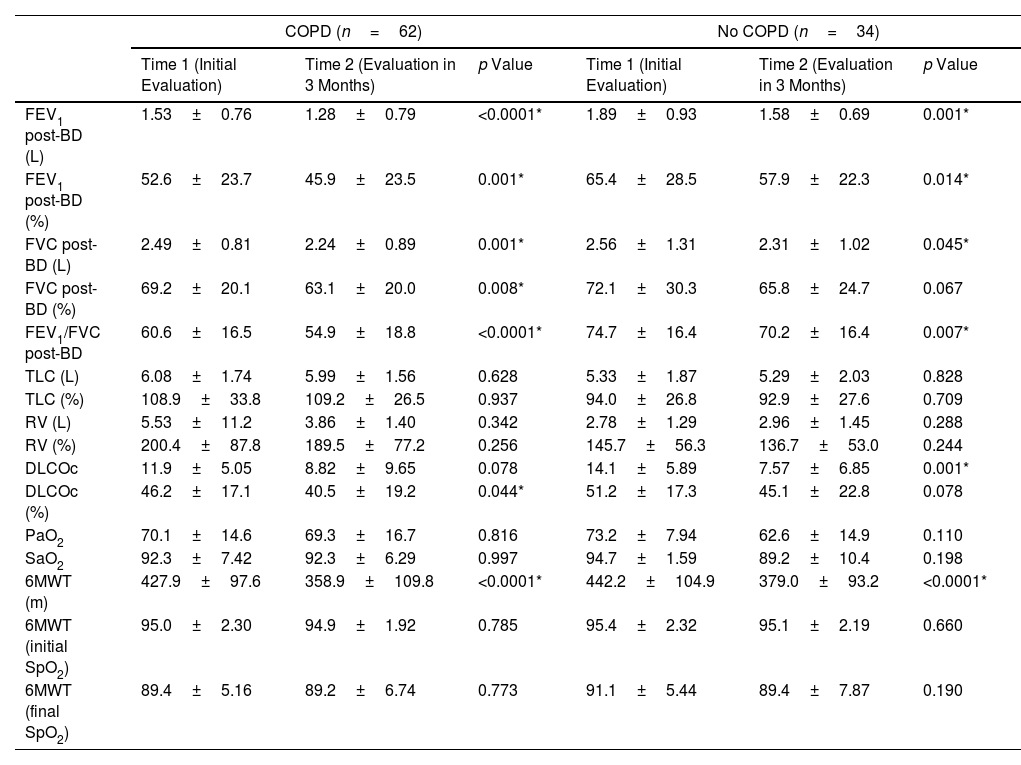
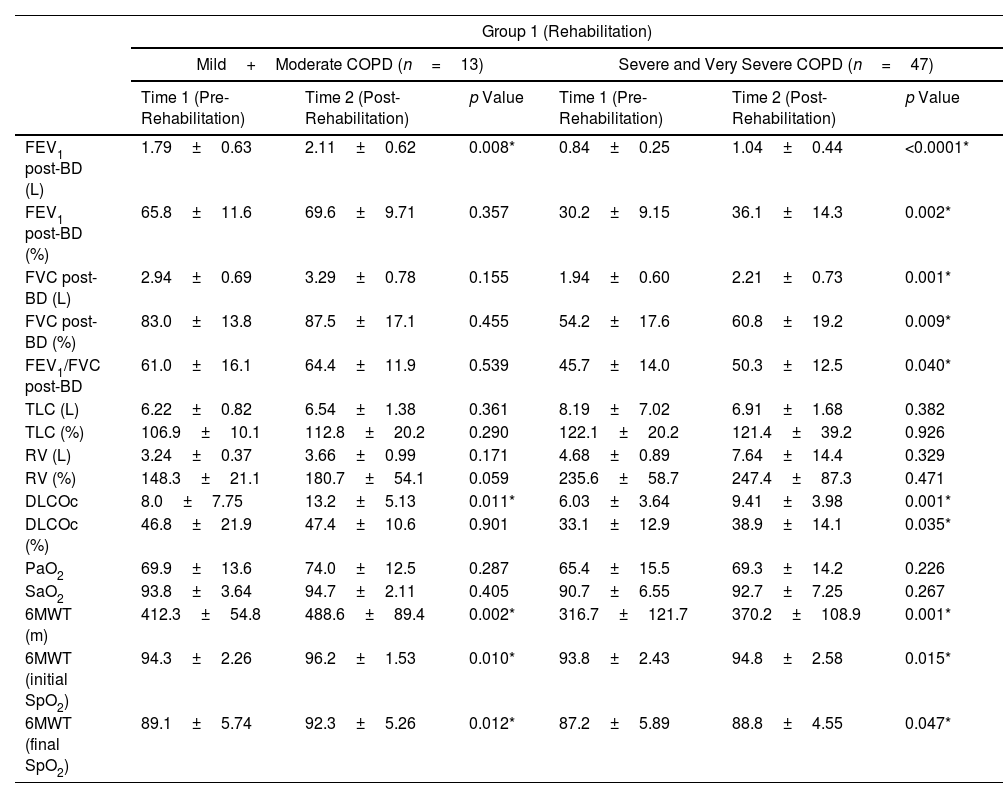
ResultsEighty-five patients were included in Group 1 and 96 patients in Group 2. Several functional parameters improved after rehabilitation, such as forced expiratory volume in one second (FEV1), forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), distance walked in 6MWT, the initial and final SaO2 at 6MWT. In Group 2, several functional parameters decreased after 3 months.
ConclusionsWe demonstrated the benefits from PRP in patients with PTLD, including improvements in lung function and in exercise capacity. National TB programs should ensure the availability of accessible and quality post-treatment PRP for PTLD patients.