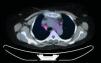
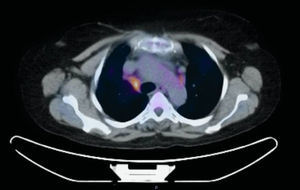
We report the case of a 58-year-old woman with a history of subclavian-jugular deep vein thrombosis in 2014, with secondary pulmonary thromboembolism and pulmonary hypertension not investigated due to refusal of consent by the patient, moderate tricuspid regurgitation and intermittent bronchial asthma. She is a native of Morocco, and last visited the country in September 2013. She lives in an urban environment, and has 2 dogs which are regularly seen by the veterinarian. No other significant epidemiological data, family history, known drug allergies, toxic habits, or occupational exposure were reported. She attended the respiratory medicine department in February 2015, referred by her primary care physician, 6–8 weeks after onset of a clinical picture of dysthermia, with undocumented fever, dyspnea on moderate exertion, cough with sparse, thick, whitish expectoration, loss of appetite, and asthenia. Physical examination revealed mild tachypnea, septic mouth with several teeth missing, no mouth ulcers, rhythmic heart sounds with no murmur, and generally reduced breath sounds with fine crackles in both lung bases. No other data of interest. Laboratory tests showed a slight increase in C-reactive protein and mild leukocytosis. No pathological findings were reported in repeat sputum samples (sputum smear and culture). Chest radiography showed general cardiomegaly and right basal interstitial-alveolar infiltrate. Chest computed tomography (CT) showed bilateral hilar and mediastinal lymphadenopathies of significant size, the latter in the lower paratracheal and subcarinal region, and alveolar infiltrate in the right lower lobe. Given the CT findings, a positron emission tomography (PET) study was performed, which confirmed increased metabolism in the lower right paratracheal region (standardized uptake value [SUV] 4.7) (Fig. 1), bilateral hilar region (SUV 2.2), and in the area of the right basal alveolar infiltrate (SUV 2.3), consistent with an infectious/inflammatory process. Flexible bronchoscopy was performed, revealing no endobronchial changes, and microbiological and cytological results were normal. Linear endobronchial ultrasound (EBUS) was subsequently performed, showing enlarged lymph nodes in level 4R, measuring 12 mm in the short axis, which was aspirated in 3 passes with a 22G cytology needle. Cytology in situ revealed ramified structures in part of the material studied. The samples were sent for cytological and microbiological analysis, and ciprofloxacin-resistant Actinomyces graevenitzii (A. graevenitzii) was isolated from all samples submitted for microbiological study. After administration of targeted antibiotic treatment with amoxicillin-clavulanate and clindamycin, the patient's clinical situation improved.
Actinomycosis is a chronic, slow-progressing granulomatous disease, caused by Gram-positive filamentous anaerobic or microaerophilic bacteria of the Actinomycetaceae family (genus Actinomyces). A. graevenitzii, specifically, was first described in 1997 by Ramos et al.1 Like other actinomycetes, A. graevenitzii forms part of the oropharyngeal flora and was initially isolated from the surface of dental implants. However, little is known about the clinical characteristics and pathogenesis of this bacteria. Pulmonary involvement occurs in up to 15% of cases of actinomycosis, thought to be mainly due to inhalation or aspiration of gastrointestinal or oropharygeal material. Infection can involve the pulmonary parenchyma, airways, pleura, mediastinum, and chest wall, causing clinical complications, such as bronchial obstruction, pleural empyema, fistulae, rib destruction, and superior vena cava syndrome.2 The most important risk factors for developing pulmonary actinomycosis include poor oropharygeal hygiene (as was the case with our patient), pre-existing dental disease, and alcoholism. Moreover, lung diseases, such as chronic obstructive pulmonary disease, bronchiectasis, chronic myobacterial disease, and aspergilloma are also considered to be risk factors due to the creation of an anaerobic environment in damaged lung tissue, which favors the growth of this bacteria.3 Immunosuppressed patients or those admitted to intensive care units are equally vulnerable to infection by opportunistic pathogens. Diagnosis can be reached with the help of endoscopic ultrasound techniques. Given the non-specific nature of the clinical and radiological characteristics of this entity, differential diagnosis with other diseases, such as lung cancer, tuberculosis,4 pneumonia, granulomatous diseases, and pulmonary abscesses, must be considered. Very few cases describing A. graevenitzii infection have been published, and this is the first known case in which diagnosis was established by linear EBUS-guided lymph node aspiration.5
Please cite this article as: Caballero Vázquez A, Cruz Rueda JJ, Ceballos Gutierrez JA. Diagnóstico mediante EBUS lineal de infección pulmonar por Actinomyces graevenitzii. Arch Bronconeumol. 2017;53:351–352.