The objective of our study was to describe the characteristics of patients diagnosed with stage I and II lung cancer in the health area of A Coruña (Galicia) and to determine their overall survival according to certain variables.
MethodsRetrospective case series in patients diagnosed between January 2011 and December 2015 with stage I and II primary lung cancer with a minimum follow-up of 18 months.
ResultsOne hundred and fifty-eight patients were included, 99 at stage I, with a median age of 69 years [range 20–90], predominantly men (81%). Adenocarcinoma was the most common histology (52.9%), followed by epidermoid carcinoma (33.1%). Asymptomatic patients (35.9%) presented more frequently in stage I. Median survival was 57 months (95% CI: 48.1–65.9), with higher survival among women, patients under 70 years of age, and those who received surgical treatment.
ConclusionsEarly-stage lung cancer in the health area of A Coruña occurs predominantly in men, in advanced age, and with adenocarcinoma histology. Survival was greater among patients with stage I disease, women, individuals aged under 70 years, and those treated surgically. Despite early diagnosis, median survival was less than 5 years.
El objetivo de nuestro estudio es detallar las características de los pacientes diagnosticados en estadios i y ii de cáncer de pulmón en el área sanitaria de A Coruña (Galicia) y conocer su supervivencia global en función de ciertas variables.
MétodosSerie de casos de carácter retrospectivo en sujetos diagnosticados entre enero de 2011 y diciembre de 2015 de cáncer pulmonar primario en estadios i y ii con un seguimiento mínimo de 18 meses.
ResultadosSe incluyeron 158 pacientes, 99 en estadio i, con una edad mediana de 69 años [rango 20–90] y mayoritariamente hombres (81%). El adenocarcinoma fue la histología más frecuente (52,9%) por encima del carcinoma epidermoide (33,1%). Los sujetos asintomáticos (35,9%) se presentaron más frecuentemente en estadio i. La mediana de supervivencia fue de 57 meses (IC 95%: 48,1–65,9), con una mayor supervivencia para el sexo femenino, los menores de 70 años y los pacientes que recibieron tratamiento quirúrgico.
ConclusionesEl cáncer de pulmón en estadios iniciales en el área de sanitaria de A Coruña presenta un predominio de hombres, edad avanzada y mayoritariamente adenocarcinomas. La supervivencia fue mayor en el estadio i, mujeres, menores de 70 años y subsidiarios de tratamiento quirúrgico. Pese a este diagnóstico precoz, la mediana de supervivencia no alcanza los 5 años.
Lung cancer (LC) is the most common global cause of cancer death in men and the third most common in women.1 It is the leading cause of cancer death in Spain, where it was estimated that 22,450 new diagnoses would be made in men and 5917 in women by 2015. While mortality in men has fallen slightly in recent years, it is increasing in women, in whom rates have almost doubled in the last decade. In Spain, LC is the third most common tumor in men and the fourth most common in women,2,3 and its impact on health and social welfare is considerable. Moreover, survival has not increased in the last few years, and the relative 5-year survival is 10.6%.4
In recent years, there has been an increase in the incidence of LC among women, and the pattern of predominant histological variants has changed due to changes in the type of tobacco smoked.5 Squamous cell carcinoma remains the most common histological type only among male smokers.6 Survival has also improved slightly in certain patient groups, primarily those with mutations that respond to targeted treatments.7,8
Data from the United States suggest that approximately 57% of LCs are diagnosed when the disease has already metastasized, and 22% of cases have regional lymph node dissemination at diagnosis. Only 16% are diagnosed when the tumor is still confined to the primary site.9 In the Corunna healthcare area, 21% of cases are diagnosed in stages I and II. Symptoms are not only rare in early-stage disease, they are also non-specific, particularly in the early stages. This leads to a significant delay in diagnosis, as other more common diseases are generally ruled out first. It is therefore unusual for LC to be diagnosed in stage I or II, and these stages are generally discovered incidentally during differential diagnosis for other diseases.10 Consequently, 5-year survival in this type of cancer, at less than 15% worldwide, remains low.5 In the United States, 5-year survival in localized disease is 55.6%.9 Screening programs among smokers have been proposed in an attempt to improve survival, but the risk–benefit ratio is unclear, and currently screening is not systematically offered by the public health system of any European country.10–15
Given the lack of evidence in stage I and II LC patients, our objective was to determine their characteristics at diagnosis and to analyze survival.
MethodsThis retrospective case series includes all patients diagnosed with stage I and II LC between January 2011 and December 2015 in the Corunna healthcare area (Galicia, Spain). Patient inclusion concluded in December 2015, to allow for a minimum follow-up of 1.5 years after diagnosis (until June 30, 2017). The reference hospital is the Complejo Hospitalario Universitario de A Coruña (CHUAC), which has a catchment area of 584,283 inhabitants.16
The study included all cases managed medically in the CHUAC LC clinic with a final diagnosis of stage IA, IB, IIA or IIB LC, according to the 7th edition of the TNM classification.17 Histopathological diagnosis was confirmed for all patients, except for 1 from whom insufficient sample material for histological study was obtained, but whose clinical and radiological picture were consistent with a diagnosis of LC. Staging was performed from clinical history, biochemistry, chest-abdomen CT and PET. EBUS/EUS was used when positive mediastinal lymphadenopathies were observed on a PET (SUV>2.5).
The following variables were collected from the electronic medical records: date of birth, sex, age at diagnosis, smoking habit (smoker/former smoker/never smoker), time since smoking cessation, tobacco consumption (pack-years), presence of COPD, reason for referral (symptoms/follow-up of respiratory disease/purely incidental), symptoms at diagnosis (none/cough/dyspnea/hemoptysis/constitutional syndrome/other), diagnostic test prompting suspicion (chest X-ray/CT/other), stage at diagnosis (IA, IB, IIA, and IIB), histological type (squamous cell/adenocarcinoma/small cell/large cell/other), treatment (surgery/chemotherapy/radiation therapy), and vital status of the patient (date of death/survival). As the reason leading to diagnosis in patients with early-stage disease was considered pertinent to this research, patients were classified as follows: (a) patients investigated for LC due to symptoms consistent with the disease, (b) patients monitored for respiratory disease, or (c) patients diagnosed with cancer by chance (incidental finding). In the latter group, the test prompting the diagnosis was not related with LC (i.e., symptoms may have been present, but this was not why the cancer was detected).
The date of death was obtained using Document Management program of the Corunna healthcare area. This system records the date of death of inhabitants in the healthcare area, although with some delay. The electronic medical records do not include the date of death if death occurs outside the hospital setting. Inclusion ended on December 31, 2015, so survival data of all patients are up-to-date. COPD was diagnosed as an FEV1/FVC ratio of <70% in a post-bronchodilator spirometry.
Bearing in mind the current debate on implementing population screening for LC, we also included a dichotomous variable: meets screening criteria according to the current US criteria, yes/no (smoker or former smoker <15 years, ≥55 and <80 years of age, and cumulative tobacco consumption of ≥30 pack-years).18
Exclusion criteria were: stage at diagnosis greater than IIB, no clinical/pathological confirmation of LC, and patients whose first consultation was in the CHUAC but who were subsequently managed in another healthcare area.
Statistical analysis was performed using SPSS 20.0. The χ2-test was used to determine a possible association between qualitative variables. Survival analysis was performed using Kaplan–Meier curves and survival functions were compared using the log-rank test to estimate p. Statistical significance was set at p<0.05.
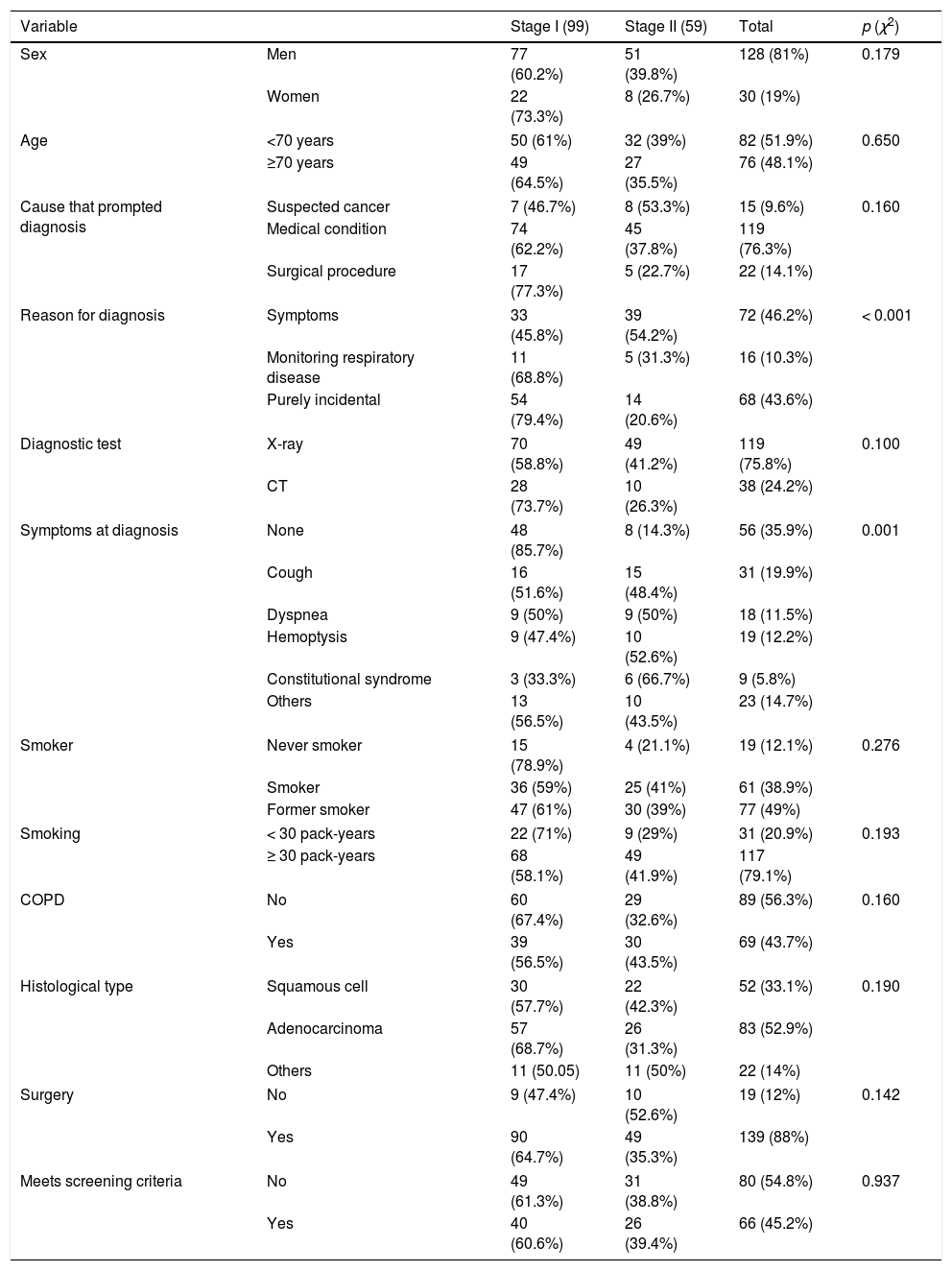
ResultsIn total, 158 patients were included in the study, 99 at stage I and 59 at stage II; 81% were men and the median age at diagnosis was 69 years (mean age, 68 years). No statistically significant differences between sex and age were found for stage at diagnosis. The predominant histology was adenocarcinoma (52.9%) (Table 1), which was the histological type most frequently diagnosed in women (73.3%) vs 48% in men, and mainly in individuals under the age of 70 years (62.2%). In patients older than 70 years, adenocarcinoma and squamous cell carcinoma occurred at similar rates.
Study population.
| Variable | Stage I (99) | Stage II (59) | Total | p (χ2) | |
|---|---|---|---|---|---|
| Sex | Men | 77 (60.2%) | 51 (39.8%) | 128 (81%) | 0.179 |
| Women | 22 (73.3%) | 8 (26.7%) | 30 (19%) | ||
| Age | <70 years | 50 (61%) | 32 (39%) | 82 (51.9%) | 0.650 |
| ≥70 years | 49 (64.5%) | 27 (35.5%) | 76 (48.1%) | ||
| Cause that prompted diagnosis | Suspected cancer | 7 (46.7%) | 8 (53.3%) | 15 (9.6%) | 0.160 |
| Medical condition | 74 (62.2%) | 45 (37.8%) | 119 (76.3%) | ||
| Surgical procedure | 17 (77.3%) | 5 (22.7%) | 22 (14.1%) | ||
| Reason for diagnosis | Symptoms | 33 (45.8%) | 39 (54.2%) | 72 (46.2%) | < 0.001 |
| Monitoring respiratory disease | 11 (68.8%) | 5 (31.3%) | 16 (10.3%) | ||
| Purely incidental | 54 (79.4%) | 14 (20.6%) | 68 (43.6%) | ||
| Diagnostic test | X-ray | 70 (58.8%) | 49 (41.2%) | 119 (75.8%) | 0.100 |
| CT | 28 (73.7%) | 10 (26.3%) | 38 (24.2%) | ||
| Symptoms at diagnosis | None | 48 (85.7%) | 8 (14.3%) | 56 (35.9%) | 0.001 |
| Cough | 16 (51.6%) | 15 (48.4%) | 31 (19.9%) | ||
| Dyspnea | 9 (50%) | 9 (50%) | 18 (11.5%) | ||
| Hemoptysis | 9 (47.4%) | 10 (52.6%) | 19 (12.2%) | ||
| Constitutional syndrome | 3 (33.3%) | 6 (66.7%) | 9 (5.8%) | ||
| Others | 13 (56.5%) | 10 (43.5%) | 23 (14.7%) | ||
| Smoker | Never smoker | 15 (78.9%) | 4 (21.1%) | 19 (12.1%) | 0.276 |
| Smoker | 36 (59%) | 25 (41%) | 61 (38.9%) | ||
| Former smoker | 47 (61%) | 30 (39%) | 77 (49%) | ||
| Smoking | < 30 pack-years | 22 (71%) | 9 (29%) | 31 (20.9%) | 0.193 |
| ≥ 30 pack-years | 68 (58.1%) | 49 (41.9%) | 117 (79.1%) | ||
| COPD | No | 60 (67.4%) | 29 (32.6%) | 89 (56.3%) | 0.160 |
| Yes | 39 (56.5%) | 30 (43.5%) | 69 (43.7%) | ||
| Histological type | Squamous cell | 30 (57.7%) | 22 (42.3%) | 52 (33.1%) | 0.190 |
| Adenocarcinoma | 57 (68.7%) | 26 (31.3%) | 83 (52.9%) | ||
| Others | 11 (50.05) | 11 (50%) | 22 (14%) | ||
| Surgery | No | 9 (47.4%) | 10 (52.6%) | 19 (12%) | 0.142 |
| Yes | 90 (64.7%) | 49 (35.3%) | 139 (88%) | ||
| Meets screening criteria | No | 49 (61.3%) | 31 (38.8%) | 80 (54.8%) | 0.937 |
| Yes | 40 (60.6%) | 26 (39.4%) | 66 (45.2%) | ||
A total of 76.3% of participants were referred to the LC fast-track clinic due to a finding encountered during the follow-up of a medical problem, and 9.6% were referred to the fast-track clinic due to a high suspicion of LC. In 43.6% of cases, LC was diagnosed incidentally, more of which were stage I than stage II. This difference was statistically significant (Table 1).
In total, 35.9% of subjects were completely asymptomatic at diagnosis, while 64.1% had some kind of symptoms associated with LC, such as cough, dyspnea, hemoptysis, constitutional syndrome, or other rarer manifestations. Only 14.3% of the asymptomatic patients were diagnosed with stage II disease (Table 1). Some incidental cases had symptoms at the time of diagnosis, but did not seek medical attention for reasons related with LC.
Never smokers represented 12.1% of the overall population, while rates among former smokers (49%) and active smokers (38.9%) were higher. One hundred and seventeen patients had a cumulative consumption of more than 30 pack-years, with no significant differences between stages I and II. The presence of COPD (43.7%) was not associated with diagnosis at a more advanced stage. Only 45.2% of patients in our series met LC screening criteria. Nineteen patients (12%) did not receive surgery, due to concomitant COPD or advanced age. The median age of unoperated patients was 79 years (10 years older than the total series), and 74% of them had COPD. In total, 53% of these patients were stage II, compared to 33% of the overall series. Four of the 5 unoperated patients without COPD were 82 years of age or more, and 1 patient aged 62 years had kidney failure, heart failure, and arteriosclerosis. In all these cases, the risk–benefit balance made surgery inadvisable.
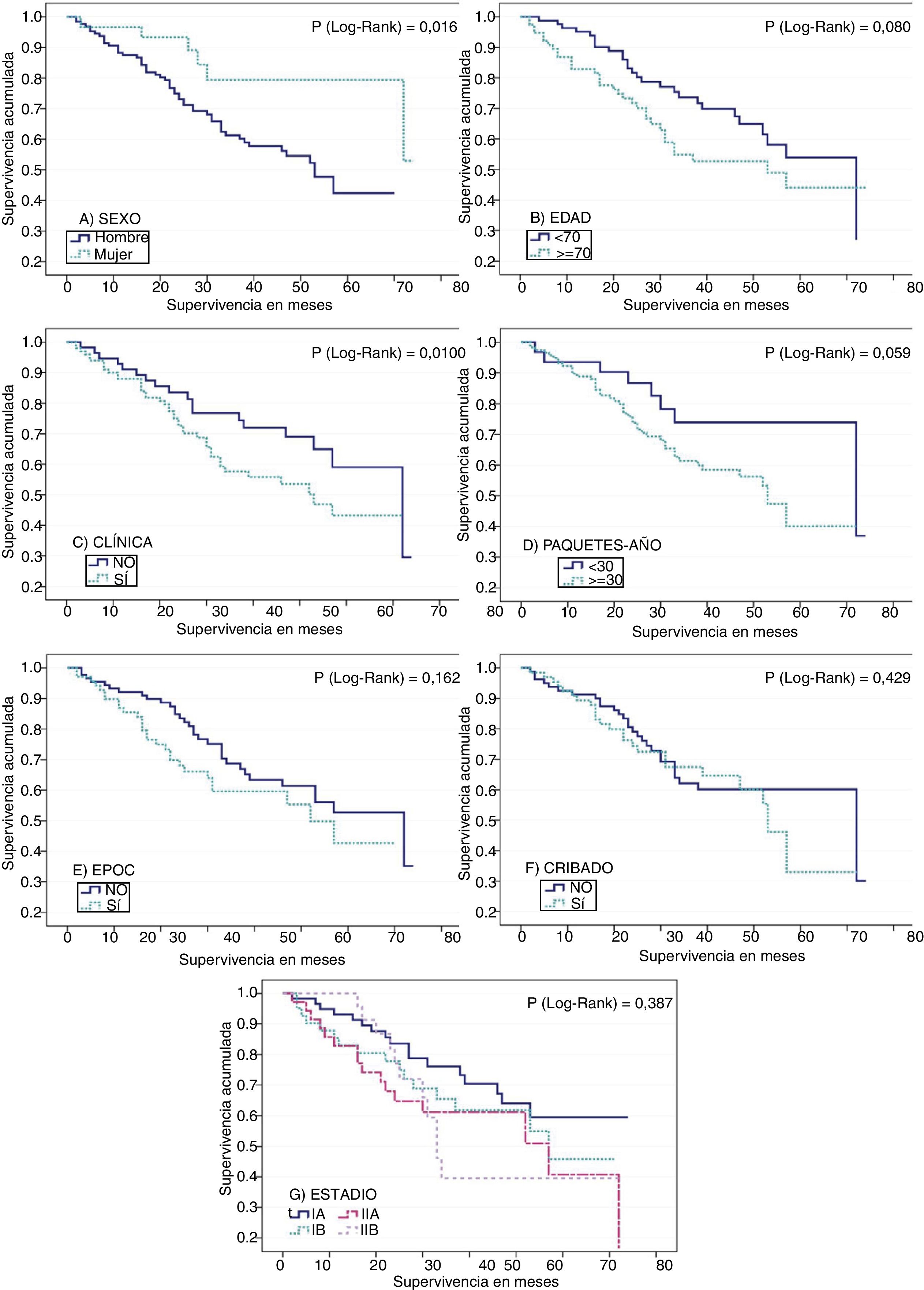
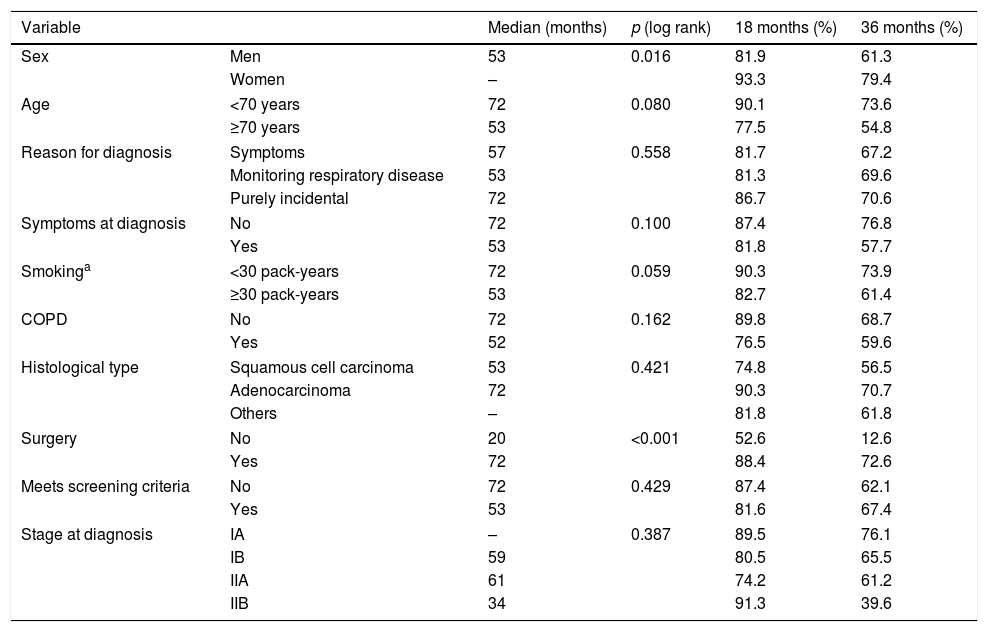
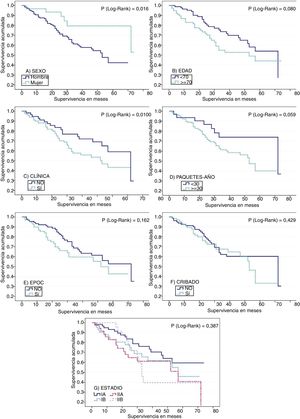
Median overall survival was 57 months (95% CI: 48.1–65.9). Survival among men was shorter than among women: 81.9% of men survived more than 18 months, compared to 93.3% of women (p=0.016). Three-year survival was 61.3% in men vs 79.4% in women. With regard to age, survival among patients aged 70 years or more was shorter than in younger subjects (p=0.08). At 3 years post-diagnosis, 54.8% of patients aged 70 years or more were alive, compared to 73.6% of the younger group (Table 2).
Survival in months by patient characteristics.
| Variable | Median (months) | p (log rank) | 18 months (%) | 36 months (%) | |
|---|---|---|---|---|---|
| Sex | Men | 53 | 0.016 | 81.9 | 61.3 |
| Women | – | 93.3 | 79.4 | ||
| Age | <70 years | 72 | 0.080 | 90.1 | 73.6 |
| ≥70 years | 53 | 77.5 | 54.8 | ||
| Reason for diagnosis | Symptoms | 57 | 0.558 | 81.7 | 67.2 |
| Monitoring respiratory disease | 53 | 81.3 | 69.6 | ||
| Purely incidental | 72 | 86.7 | 70.6 | ||
| Symptoms at diagnosis | No | 72 | 0.100 | 87.4 | 76.8 |
| Yes | 53 | 81.8 | 57.7 | ||
| Smokinga | <30 pack-years | 72 | 0.059 | 90.3 | 73.9 |
| ≥30 pack-years | 53 | 82.7 | 61.4 | ||
| COPD | No | 72 | 0.162 | 89.8 | 68.7 |
| Yes | 52 | 76.5 | 59.6 | ||
| Histological type | Squamous cell carcinoma | 53 | 0.421 | 74.8 | 56.5 |
| Adenocarcinoma | 72 | 90.3 | 70.7 | ||
| Others | – | 81.8 | 61.8 | ||
| Surgery | No | 20 | <0.001 | 52.6 | 12.6 |
| Yes | 72 | 88.4 | 72.6 | ||
| Meets screening criteria | No | 72 | 0.429 | 87.4 | 62.1 |
| Yes | 53 | 81.6 | 67.4 | ||
| Stage at diagnosis | IA | – | 0.387 | 89.5 | 76.1 |
| IB | 59 | 80.5 | 65.5 | ||
| IIA | 61 | 74.2 | 61.2 | ||
| IIB | 34 | 91.3 | 39.6 | ||
No significant differences were found in median survival between symptomatic subjects, those who were being followed up for respiratory disease, and those with an incidental finding of suspected LC on an imaging test. However, median survival varied among groups, being longer in incidental cases, with a median survival of 72 months compared to 57 months in symptomatic patients, and 53 months in patients in clinical follow-up. This is also consistent with the data obtained when patients who were asymptomatic at the time of diagnosis (median survival of 72 months) were compared with patients with symptoms of LC (53 months), although the difference did not reach statistical significance (Table 2 and Fig. 1).
Survival functions for (A) sex, (B) age (older or younger than 70 years at diagnosis), (C) clinical symptoms suggestive of lung cancer, (D) tobacco consumption in pack-years, (E) presence or absence of COPD, (F) compliance or non-compliance with criteria for lung cancer screening, and (G) stage at diagnosis.
Survival was shorter in smokers of ≥30 pack-years (53 months) than in those with a consumption of <30 pack-years (72 months). These differences approached statistical significance. Patients with COPD also died earlier (18-month survival of 76.5%) than patients without respiratory disease (18-month survival of 89.8%). The difference between the survival data of these groups 3 years after diagnosis was rather less marked (Table 2 and Fig. 1). Median survival among patients who did not undergo surgery was much shorter (20 vs 72 months) (Table 2).
Survival at 18 months was better in the group who did not meet screening criteria, but the opposite is the case for a 3-year survival (Fig. 1F).
DiscussionThese results show that survival in stage I and II LC is still low. Half of our patients died within 57 months of diagnosis (median survival of less than 5 years); these data are a slight improvement on US figures.9 Surgery probably cannot be considered a curative treatment, although it clearly has a crucial effect on survival. These poor outcomes are remarkable if we remember that 5-year survival after early-stage breast cancer is around 90%.19
The epidemiology of LC is changing. In Spain, LC mortality in women has doubled in the last 10 years,20 a trend that is reflected in our series, where the male:female ratio is 4.3:1, lower than that reported in previous series.21 These data are in line with changes in the epidemiological distribution of LC by sex in Spain and Europe.22 With regard to age, our median of 69 years is similar to that of the rest of Spain.23
Detecting LC at an early stage is difficult, particularly because symptoms tend to be slow to develop. Even so, in this study, only 35.9% of cases were asymptomatic at diagnosis, despite being early-stage disease. We should highlight the fact that a large number of patients had non-specific symptoms, such as cough (19.9%) and dyspnea (11.5%). Almost half of the stage I cases were asymptomatic at diagnosis. As the size and stage of the tumor advance, symptoms become more marked.24 We should also mention incidental diagnoses, since these chance findings accounted for 43.6% of all patients, and were more frequently detected in stage I. Incidental diagnoses may be more frequent than diagnoses in asymptomatic patients because the most common symptoms of LC are non-specific, and while some subjects had symptoms at diagnosis, this was not the reason for their referral to the LC clinic.
In total, 79.1% of patients had smoked more than 30 pack-years, and 87.9% were smokers or former smokers. More stage I than stage II disease was detected in heavy smokers, possibly because of the high percentage of patients diagnosed during monitoring for another medical condition (76.3%). In addition, LC may remain longer at stage I than at stage II, thus increasing the window of opportunity for tumor detection and facilitating earlier diagnosis.
In a study conducted in the same healthcare area in 2007, the most common histology was squamous cell carcinoma (48.7%).25 Our study, however, clearly reflects epidemiological changes, insofar as the most predominant histological type was adenocarcinoma, accounting for 52.9% of the overall findings.
Stage at diagnosis is the most important prognostic variable for survival, although advanced age or male sex are factors that also impact significantly on mortality.26 Sex and age play an important role in survival in early-stage disease. Only 54.8% of patients aged 70 years or older were still alive 36 months after diagnosis, compared to 73.6% of patients younger than 70. This may be related with a better response to surgery or drug treatment, since older patients have more comorbidities and higher mortality rates associated with surgery than younger patients.
Surgery is the optimal treatment for early-stage LC. An analysis by the Danish Lung Cancer Registry found that survival in early-stage disease was around 80% in the first year after surgery.27 A very recent American study found that 5-year survival in stage I patients who met the inclusion criteria for the National Lung Screening Trial (NLST) was 63% compared to 47% among those who did not meet these criteria.28 Our study results are somewhat better, revealing a median survival of 57 months (almost 5 years) in patients with stage I and II disease, including those who did not meet NSLT criteria. Survival data from the UK show 1-year survival rates of 85.1% and 71% in stage I and II disease, respectively.29 Survival in our series was as high as 88.4% (Table 2). Considering that we assessed vital status at 18 months, these outcomes seem to be somewhat better than those of the Danish study. It should be noted that our hospital is a reference center for LC thoracic surgery and many of our patients underwent uniportal VATS procedures, which may have had a positive impact on survival.
COPD, as a risk factor for the development of postoperative pulmonary complications, also affected survival.30 In fact, patients with advanced COPD are not candidates for surgery. High tobacco consumption and COPD were seen to reduce life expectancy in patients included in this study. Although the data analysis failed to reach statistical significance, Fig. 1 shows differences in the survival curves.
Stage at diagnosis is one of the most important factors affecting survival in a patient with LC.31 We found statistically significantly improved survival when LC was diagnosed in asymptomatic patients or incidentally, and in both cases this benefit is more frequently associated with stage I disease. We also found that more invasive disease tended to result in poorer survival, since 3-year survival in stage IA patients was 76.1%, but 39.6% in stage IIB.
In our study, we found no differences in survival between patients who met screening criteria and those who did not. It is interesting to note that the cases in our series should, in principle, be those most frequently detected in an LC screening program. However, only 45% of our cases met the screening criteria, so the remaining 55% would not, in theory, have been detected by a screening program. Tanner et al.28 reported similar non-compliance with screening inclusion criteria in stage I patients.
Our study has two major limitations. Firstly, it is a retrospective case review, so detailed information about the cause of death could not be collected, as little information is provided in the electronic medical record if death occurred outside the hospital setting. As a result, the dependent variable from which survival was calculated was all-cause death, and we were unable to collect data on mortality attributable to a possible pulmonary recurrence or the appearance of a new primary tumor. The second limitation is that cases of LC diagnosed in 2011 and 2012 could have been lost. During this period, the CHUAC was in the process of implementing its electronic clinical record system, and data from some LC consultations were not entered.32 This suggests that a small percentage of cases may have been missed between the years 2011 and 2012.
The strengths of this study are its long inclusion period and its relatively large sample size, given that LC is rarely diagnosed in early stages. Moreover, consecutive sampling means that the cases are representative of patients seen in our setting.
ConclusionsIn conclusion, the distribution of LC in the Corunna healthcare area, in terms of sex, age, and histological type, is in line with current epidemiological patterns. Variables with the greatest impact on survival are stage at diagnosis, sex, age, and surgical treatment. Despite early-stage diagnosis and the high number of incidental findings, the proportion of completely asymptomatic patients was low, and median survival of patients diagnosed with stage I and II disease was less than 5 years. Finally, only 45% of the cases met the US criteria for screening, and survival in patients who did meet these criteria was similar to those who did not.
Conflict of interestThe authors state that they have no conflict of interests.
We thank all those who, in one way or other, have contributed to the conduct of this study.
Please cite this article as: Pérez-Martínez O, Vidal-García I, Montero-Martínez C, Provencio M, Ruano-Ravina A. Características al diagnóstico y supervivencia de estadios i y ii de cáncer de pulmón. Arch Bronconeumol. 2018;54:420–426.