An increase in respiratory isolates of Exophiala dermatitidis has been described in recent years in patients with cystic fibrosis (CF). We report the case of a CF patient with chronic E. dermatitidis bronchial infection.
This was a 21-year-old woman who had been diagnosed with genotype F508del/3849+1G>A CF at the age of 3 months. Chest computed tomography (CT) revealed multiple cylindrical, cystic, and string-of-pearls bronchiectasis in both lungs. Spirometry showed moderate-severe pulmonary obstruction with a forced expiratory volume in 1 second (FEV1) of 1680ml (53% predicted). Pancreatic insufficiency and intermittent bronchial infection caused by methicillin-sensitive Staphylococcus aureus, Pseudomonas aeruginosa, and Achromobacter xylosoxidans were detected. The patient had shown declining lung function, and in recent years only E. dermatitidis was isolated from sputum microbiology studies. Given her clinical deterioration and the absence of bacterial growth, we performed a bronchoscopy, obtaining bronchial aspirate (BAS) and bronchoalveolar lavage (BAL) samples. Selective media, Sabouraud agar and blood agar, were seeded quantitatively and incubated for 5 days. MALDI-TOF mass spectrometry was used for the identification of the different pathogens. E. dermatitidis grew from both BAL and BAS, and antibiotic sensitivity testing was performed with amphotericin B and voriconazole using the Etest® method, obtaining MICs of 0.1 and 0.023, respectively. Treatment with oral voriconazole 300mg/12h began, but adverse effects (hallucinations and altered liver profile) led the dose to be reduced to the maximum tolerable level of 100mg/12h. During follow-up, the patient has shown important clinical improvement and reduced exacerbations, despite persistent isolation of the fungus.
The prevalence of E. dermatitidis in CF patients varies between 2% and 15%.1 This may be due to the lack of standardized procedures for the detection of this organism in sputum samples. E. dermatitidis is a slow-growing opportunistic fungus that is not ubiquitous, and as such is generally an uncommon contaminant in microbiology laboratories. It is mostly detected in patients with CF, so isolation in a non-CF patient should prompt suspicion.2
It was first described in 1990,3 and some cases have been published since then. In 2010, the first case of pigmented sputum was described, with the black flecks being attributed to fungal hyphae.4 In 2017, Grenouillet et al. published 2 cases of patients with bronchiectasis and chronic persistent E. dermatitidis colonization which led to the diagnosis of CF.2
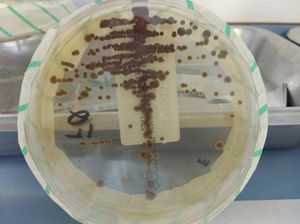
For the definitive diagnosis of this fungus, the sample must be cultured in Sabouraud agar, incubated at room temperature or 30°C, and repeated isolates must be obtained. Cultures must be observed for 3–4 weeks, although colonies are usually detected in less than 7 days.5 The colonies are small at first, and over time increase in size and acquire a characteristic intense olive black or dark brown color (Fig. 1). However, E. dermatitidis isolation is sometimes complicated, and the use of appropriate media, such as erythritol-chloramphenicol agar (ECA) can increase the recovery rate.6 Molecular techniques (LAMP or reverse hybridization) can be powerful alternatives to culture media, increasing the rate of detection in sputum samples.6
In patients with CF, chronic or intermittent E. dermatitidis isolation usually has no clinical repercussions, although some cases have been reported, such as that of a child with CF who presented symptoms of dyspnea due to E. dermatitidis pneumonia.7 Two prospective studies in a Swedish cohort of 98 CF patients over 12 years of age found E. dermatitidis or elevated serum levels of IgG antibodies to E. dermatitidis to be associated with pancreatic insufficiency, more frequent colonization by non-tuberculous mycobacteria, increased inflammatory markers, requirements for more frequent intravenous antibiotic treatment, and lower FEV1.8 Although the clinical impact of this pathogen is still pending investigation, its presence in the respiratory tract must be monitored as it is currently considered to be an emerging opportunistic pathogen in CF.9
Please cite this article as: Martín Ramírez A, Erro Iribarren M, Buendía Moreno B, María Girón R. Deterioro clínico por Exophiala dermatitidis en un paciente con fibrosis quística. Arch Bronconeumol. 2019;55:162–163.