Several studies have shown that the interaction between chronic obstructive pulmonary disease (COPD) and cardiovascular comorbidity is complex and bidirectional, since each of these diseases complicates the prognosis of the other.
Recent advances in imaging technology have led to better characterization of cardiac chambers and allowed the relationship between certain cardiac function parameters and COPD clinical and functional variables to be explored.
Although cardiac abnormalities in COPD have been mainly associated with the right ventricle, several studies have reported that the left ventricle may also be affected in this disease. A better understanding of the mechanisms involved and their clinical implications will establish diagnostic and therapeutic strategies for patients with both these conditions.
Numerosos estudios han puesto de manifiesto que la interacción entre la enfermedad pulmonar obstructiva crónica (EPOC) y la comorbilidad cardiovascular es compleja y bidireccional, puesto que cada una de estas entidades complica el pronóstico de la otra.
El avance en las técnicas de imagen ha dado paso a una mejor caracterización de las cavidades cardíacas, hecho que ha permitido el estudio de la relación que existen entre ciertos parámetros de función cardíaca con variables clínicas y funcionales en la EPOC.
A pesar de que las alteraciones cardíacas en la EPOC han sido adscritas fundamentalmente al ventrículo derecho, diversos estudios han descrito que el ventrículo izquierdo también se puede afectar en esta enfermedad. Una mejor compresión de los mecanismos involucrados y de sus implicaciones clínicas permitirá establecer estrategias de abordaje diagnóstico y terapéutico en los pacientes donde coexistan estas 2 entidades.
The anatomical and functional relationship between the heart and lungs is so close that dysfunction of one of these systems can affect the other.1 There are neurological, humoral and mechanical interactions between both organs, and various mechanisms that lead to structural or functional ventricular alterations can coexist in patients with respiratory disease. Several studies have shown that cardiovascular events are more common in patients with chronic obstructive pulmonary disease (COPD) compared to smokers without the disease.2–4 However, whether this is simply due to the higher prevalence of traditional cardiovascular risk factors (CVRF) (hypertension [HT], diabetes mellitus, reduced physical activity and dyslipidemia)5 in COPD patients, or whether there is a particular pathophysiological connection is still widely debated. While some authors propose systemic inflammation as the etiological pathway to atherosclerosis, recent studies indicate that sustained systemic inflammation occurs in only a proportion of patients with COPD.6 Thus, the association between cardiovascular diseases (CVD) and COPD is much more complex, and may involve other factors: biological (hypoxemia, endothelial dysfunction, increased platelet activation, arterial stiffness),7–9 mechanical and/or functional (deterioration in the forced expiratory volume in the first second, emphysema, hyperinflation),10,11 neurohumoral (excess sympathetic nerve activity)12 and genetic (polymorphisms of the metalloproteinases, telomere shortening).13,14
There is growing interest in the as yet poorly characterized contribution of cardiovascular factors such as dyspnea and exercise intolerance to the symptomatology of COPD. Indeed, various studies with large patient cohorts have identified a cardiovascular phenotype in COPD that presents with a different clinical course and prognosis.15–17
The cardiac abnormality related with COPD has traditionally been right ventricular (RV) dysfunction, despite publications in the last century already reporting pathological left ventricle (LV) changes found in the autopsied hearts of COPD patients.18 At present, thanks to advances in imaging techniques, various LV abnormalities in these patients that appear to affect certain clinical and functional variables of the disease have been verified. In this review, we will analyze the mechanisms linking LV dysfunction and COPD, their manifestation in imaging studies, and their clinical consequences.
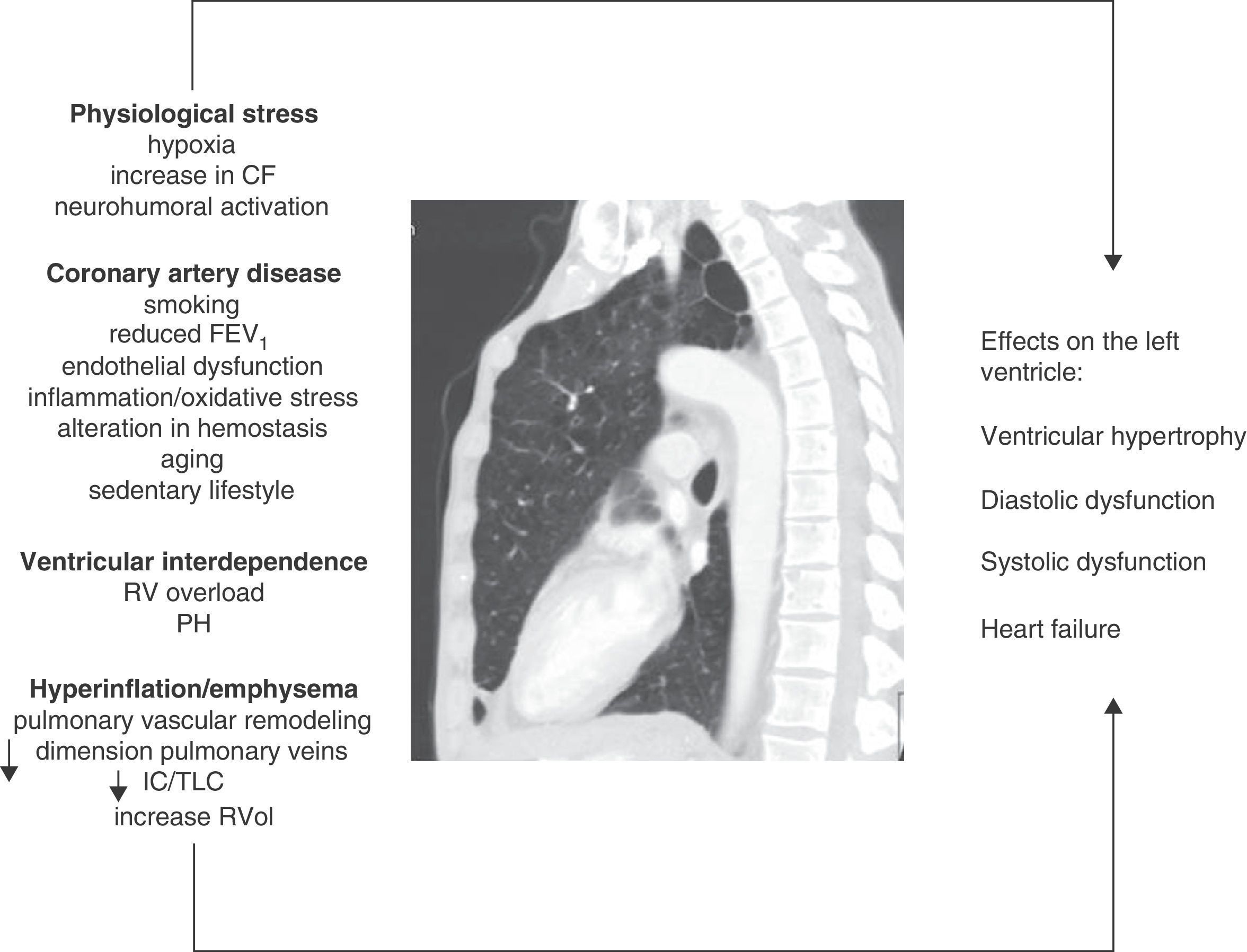
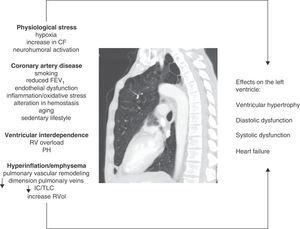
Mechanisms InvolvedPhysiological StressPatients with COPD can have sustained (patients with chronic respiratory failure), or intermittent hypoxia (during exercise, exacerbations, or during sleep). Hypoxia can cause abnormalities in ventricular relaxation and contraction due to changes in the myocyte cell metabolism.19 It can also affect the pathogenesis of atherosclerosis by various mechanisms, including increased vascular and systemic inflammation, elevated C-reactive protein and increased oxidative stress.20,21 Furthermore, it can induce hemodynamic stress by increasing the heart rate and activating the sympathetic nervous system.22,23 Finally, hypoxia is involved in pulmonary vascular remodeling that increases pulmonary vascular resistance, which may negatively affect LV diastolic filling by the phenomenon of ventricular interdependence, described below.
Coronary Artery DiseaseCoronary artery disease (CAD) or atherosclerosis is the end result of the accumulation of atheroma plaques on the walls of the coronary arteries. Numerous epidemiological studies have shown that COPD patients have a high risk of developing CAD, with its inherent complications (ischemic heart disease, stroke, sudden death), and that this risk increases during exacerbations.24–26 Some studies have found this association to be independent of smoking and other confounding factors, such as age. Sub-clinical atherosclerosis (the “early” phase of CAD) has also been described in smokers with airflow limitation and in emphysema patients.27,28
The etiology of CAD and COPD is complex and multifactorial, since they share common etiological factors apart from smoking (release of endothelial microparticles, changes in hemostasis and oxidative stress, among others).29–31
The precise prevalence of CAD in COPD is not known; estimates published to date vary widely (4.7%–60%).32 Nevertheless, data from population studies indicate that it could be high.33–35
Other evidence that highlights the close relationship between CAD and COPD is the role of the latter as an independent factor for poor progress and mortality following coronary revascularisation.36–38 COPD, together with 5 other major clinical variables including age, sex and LV ejection fraction (LVEF), is a predictor of mortality 4 years after revascularization in the SYNTAX score II (an angiographic grading tool to determine the complexity of CAD that helps clinicians decide the optimum revascularization method in patients with complex CAD).39
CAD can affect myocardial relaxation by decreasing arterial distensibility, and increasing central arterial pressure and LV afterload,40 while subclinical atherosclerosis has been negatively associated with diastolic dysfunction parameters due to altered coronary reserve.29
Ventricular InterdependenceRight ventricular function and pulmonary hypertension (PH), usually only mild to moderate, are common in COPD. Both pulmonary vascular changes and pathological changes in the RV have been found even in early stages of the disease.7,41 Ventricular interdependence describes the phenomenon in which both RV pressure and volume overload cause the interventricular septum to shift toward the LV, modifying its geometry (“D-shape”). The pathophysiological mechanisms involved are summarized in Fig. 1. Dilatation of the RV also increases the constrictive effect of the pericardium, all of which can result in a reduction in the distensibility and filling of the LV.42 This mechanism may explain why a preserved ejection fraction can be observed in the LV, despite a sub-optimal filling phase.
Figure summarizing the pathophysiological mechanisms involved and their effects on the left ventricle in COPD. CF: cardiac frequency; FEV1: forced expiratory volume in the first second; IC/TLC: ratio between the inspiratory capacity and total lung capacity; PH: pulmonary hypertension; RV: right ventricle; RVol: residual volume.
Thanks to recent advances and the widespread availability of imaging techniques, the effect of emphysema and hyperinflation on LV dysfunction (LVD) is now understood in greater detail. Ten years ago, Jörgensen et al.43–45 hypothesized that hyperinflated lungs and high intrinsic positive end-expiratory pressure will decrease intrathoracic blood volume and ventricular preload, resulting in “hypovolemic diastole”. The authors showed through various studies that patients with severe emphysema had various functional and hemodynamic alterations, such as a decrease in the end-systolic and end-diastolic volumes of the LV, lower cardiac index and lower stroke volume index compared to a control group, and that furthermore, these parameters could improve after volume reduction surgery.47
In this respect, Watz et al.,11 in an echocardiographic study of 138 patients with COPD of varying severity, observed that pulmonary hyperinflation (measured by the inspiratory capacity/total lung capacity [IC/TLC] ratio) was more strongly correlated with the decreased size of the heart chambers and impaired LV diastolic filling pattern than with airflow obstruction or the carbon monoxide diffusing capacity.
Barr et al.10 provided solid evidence of the effect of emphysema on ventricular filling in the MESA study, an extensive population study in patients without CVRF. Emphysema detected by computed tomography (CT) and airflow obstruction were linearly and inversely correlated with the reduction in LV end-diastolic volume, systolic volume and cardiac output measured by magnetic resonance imaging (MRI). These associations were of greater magnitude among the active smokers than among former and never-smokers. These findings indicate that even in the early stages of COPD, the systolic volume and size of the LV are affected.46 In 2 MESA sub-studies, Smith et al.47 described an association between 2 pulmonary hyperinflation parameters (residual volume and the residual volume/TLC ratio) with a larger LV mass in 119 patients with COPD, while another study by the same group showed that the percentage of emphysema was inversely correlated with the diameter of the pulmonary veins,48 which were narrower in COPD patients (GOLD I–III) than in controls, although the difference was not statistically significant (P=.06). This structural alteration may also be detrimental to LV filling.
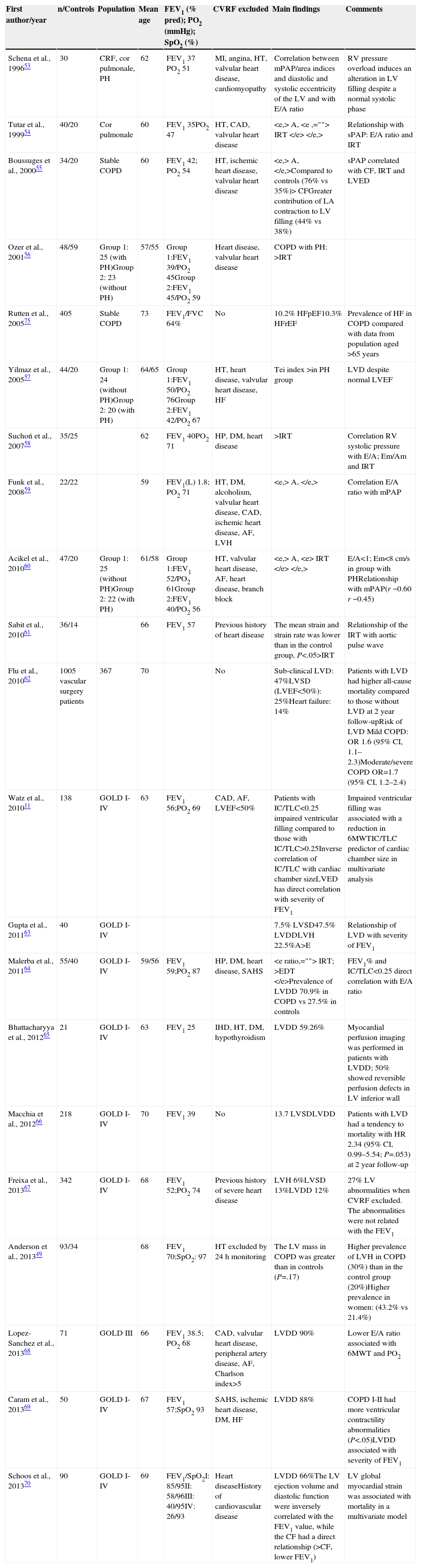
Left Ventricular Defects in Chronic Obstructive Pulmonary Disease and Their Clinical ConsequencesThe morphological description of the heart chambers in COPD using echocardiography raises the issue of suboptimal quality if hyperinflation and diaphragmatic flattening coexist. Despite this, various echocardiographic series have been published in the last 20 years showing alterations in structural and functional parameters over the entire spectrum of disease severity (Table 1). The variability in the findings reported depends, in some cases, on the inclusion of “selected” patients (no CVRF except smoking), the presence of associated PH, the setting (specialist clinic/primary care) and the degree of airflow obstruction. There are also studies with other imaging techniques that are less commonly used in routine clinical practice, such as MRI and nuclear medicine techniques.
Left Ventricle Echocardiography Studies in COPD Patients.
| First author/year | n/Controls | Population | Mean age | FEV1 (% pred); PO2 (mmHg); SpO2 (%) | CVRF excluded | Main findings | Comments |
|---|---|---|---|---|---|---|---|
| Schena et al., 199653 | 30 | CRF, cor pulmonale, PH | 62 | FEV1 37 PO2 51 | MI, angina, HT, valvular heart disease, cardiomyopathy | Correlation between mPAP/area indices and diastolic and systolic eccentricity of the LV and with E/A ratio | RV pressure overload induces an alteration in LV filling despite a normal systolic phase |
| Tutar et al., 199954 | 40/20 | Cor pulmonale | 60 | FEV1 35PO2 47 | HT, CAD, valvular heart disease | Relationship with sPAP: E/A ratio and IRT | |
| Boussuges et al., 200055 | 34/20 | Stable COPD | 60 | FEV1 42; PO2 54 | HT, ischemic heart disease, valvular heart disease | sPAP correlated with CF, IRT and LVED | |
| Ozer et al., 200156 | 48/59 | Group 1: 25 (with PH)Group 2: 23 (without PH) | 57/55 | Group 1:FEV1 39/PO2 45Group 2:FEV1 45/PO2 59 | Heart disease, valvular heart disease | COPD with PH: >IRT | |
| Rutten et al., 200575 | 405 | Stable COPD | 73 | FEV1/FVC 64% | No | 10.2% HFpEF10.3% HFrEF | Prevalence of HF in COPD compared with data from population aged >65 years |
| Yilmaz et al., 200557 | 44/20 | Group 1: 24 (without PH)Group 2: 20 (with PH) | 64/65 | Group 1:FEV1 50/PO2 76Group 2:FEV1 42/PO2 67 | HT, heart disease, valvular heart disease, HF | Tei index >in PH group | LVD despite normal LVEF |
| Suchoń et al., 200758 | 35/25 | 62 | FEV1 40PO2 71 | HP, DM, heart disease | >IRT | Correlation RV systolic pressure with E/A; Em/Am and IRT | |
| Funk et al., 200859 | 22/22 | 59 | FEV1(L) 1.8; PO2 71 | HT, DM, alcoholism, valvular heart disease, CAD, ischemic heart disease, AF, LVH | Correlation E/A ratio with mPAP | ||
| Acikel et al., 201060 | 47/20 | Group 1: 25 (without PH)Group 2: 22 (with PH) | 61/58 | Group 1:FEV1 52/PO2 61Group 2:FEV1 40/PO2 56 | HT, valvular heart disease, AF, heart disease, branch block | E/A<1; Em<8cm/s in group with PHRelationship with mPAP(r −0.60 r −0.45) | |
| Sabit et al., 201061 | 36/14 | 66 | FEV1 57 | Previous history of heart disease | The mean strain and strain rate was lower than in the control group, P<.05>IRT | Relationship of the IRT with aortic pulse wave | |
| Flu et al., 201062 | 1005 vascular surgery patients | 367 | 70 | No | Sub-clinical LVD: 47%LVSD (LVEF<50%): 25%Heart failure: 14% | Patients with LVD had higher all-cause mortality compared to those without LVD at 2 year follow-upRisk of LVD Mild COPD: OR 1.6 (95% CI, 1.1–2.3)Moderate/severe COPD OR=1.7 (95% CI, 1.2–2.4) | |
| Watz et al., 201011 | 138 | GOLD I-IV | 63 | FEV1 56;PO2 69 | CAD, AF, LVEF<50% | Patients with IC/TLC<0.25 impaired ventricular filling compared to those with IC/TLC>0.25Inverse correlation of IC/TLC with cardiac chamber sizeLVED has direct correlation with severity of FEV1 | Impaired ventricular filling was associated with a reduction in 6MWTIC/TLC predictor of cardiac chamber size in multivariate analysis |
| Gupta et al., 201163 | 40 | GOLD I-IV | 7.5% LVSD47.5% LVDDLVH 22.5%A>E | Relationship of LVD with severity of FEV1 | |||
| Malerba et al., 201164 | 55/40 | GOLD I-IV | 59/56 | FEV1 59;PO2 87 | HP, DM, heart disease, SAHS | FEV1% and IC/TLC<0.25 direct correlation with E/A ratio | |
| Bhattacharyya et al., 201265 | 21 | GOLD I-IV | 63 | FEV1 25 | IHD, HT, DM, hypothyroidism | LVDD 59.26% | Myocardial perfusion imaging was performed in patients with LVDD; 50% showed reversible perfusion defects in LV inferior wall |
| Macchia et al., 201266 | 218 | GOLD I-IV | 70 | FEV1 39 | No | 13.7 LVSDLVDD | Patients with LVD had a tendency to mortality with HR 2.34 (95% CI, 0.99–5.54; P=.053) at 2 year follow-up |
| Freixa et al., 201367 | 342 | GOLD I-IV | 68 | FEV1 52;PO2 74 | Previous history of severe heart disease | LVH 6%LVSD 13%LVDD 12% | 27% LV abnormalities when CVRF excluded. The abnormalities were not related with the FEV1 |
| Anderson et al., 201349 | 93/34 | 68 | FEV1 70;SpO2: 97 | HT excluded by 24h monitoring | The LV mass in COPD was greater than in controls (P=.17) | Higher prevalence of LVH in COPD (30%) than in the control group (20%)Higher prevalence in women: (43.2% vs 21.4%) | |
| Lopez-Sanchez et al., 201368 | 71 | GOLD III | 66 | FEV1 38.5; PO2 68 | CAD, valvular heart disease, peripheral artery disease, AF, Charlson index>5 | LVDD 90% | Lower E/A ratio associated with 6MWT and PO2 |
| Caram et al., 201369 | 50 | GOLD I-IV | 67 | FEV1 57;SpO2 93 | SAHS, ischemic heart disease, DM, HF | LVDD 88% | COPD I-II had more ventricular contractility abnormalities (P<.05)LVDD associated with severity of FEV1 |
| Schoos et al., 201370 | 90 | GOLD I-IV | 69 | FEV1/SpO2I: 85/95II: 58/96III: 40/95IV: 26/93 | Heart diseaseHistory of cardiovascular disease | LVDD 66%The LV ejection volume and diastolic function were inversely correlated with the FEV1 value, while the CF had a direct relationship (>CF, lower FEV1) | LV global myocardial strain was associated with mortality in a multivariate model |
A: atrial contraction wave on the mitral Doppler flow; LA: left atrium; Am: diastolic velocity of the myocardium obtained by tissue Doppler during atrial contraction; IHD: ischemic heart disease; LVDD: left ventricular diastolic dysfunction; DM: diabetes mellitus; LVSD: left ventricular systolic dysfunction; LVED: left ventricular end-diastolic diameter; LVD: left ventricular dysfunction; E: early diastolic filling wave on the mitral Doppler flow; CAD: coronary artery disease; EDT: early deceleration time of the early ventricular filling wave; Em: diastolic velocity of the myocardium obtained by tissue Doppler during early filling; E/A: ratio between the E wave and the A wave on mitral Doppler flow; Em/Am: ratio between the Em wave and the Am wave; AF: atrial fibrillation; CF: cardiac frequency; FEV1: forced expiratory volume in the first second; LVEF: left ventricular ejection fraction; CVRF: cardiovascular risk factors; PH: pulmonary hypertension; HR: hazard ratio; HT: systemic arterial hypertension; LVH: left ventricular hypertrophy; HF: heart failure; IC/TLC: ratio between the inspiratory capacity and total lung capacity; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced rejection fraction; MI: myocardial infarction; CRF: chronic respiratory failure; OR: odds ratio; mPAP: mean pulmonary artery pressure; sPAP: systolic pulmonary artery pressure; PO2: arterial oxygen pressure; IRT: left ventricular isovolumetric relaxation time; SAHS: sleep apnea–hypopnea syndrome; SpO2: arterial oxygen saturation; 6MWT: 6-min walk test; RV: right ventricle; LV: left ventricle.
As shown above, ventriculography and autopsy studies in patients with chronic bronchitis and emphysema show LV hypertrophy (LVH) and increased ventricular mass.18 In addition to the study by Smith et al.,47 which describes an association between pulmonary hyperinflation and LV mass independent of other CVRF, Anderson et al.,49 using echocardiography, reported a prevalence of LVH of 21.4% in men and 43.2% in women with normoxemic COPD and no underlying PH; the LV mass was significantly larger than that of controls. These findings indicate an independent effect of COPD on LVH that is not related with PH. The authors suggested sympathetic activation as the possible mechanism, mainly through the renin–angiotensin–aldosterone system.
LVH is a pivotal factor in cardiovascular events, and preventive treatment has been shown to reduce cardiovascular morbidity and mortality to a large extent.50 LVH is considered an arrhythmogenic factor and can result in the eventual onset of ventricular dysfunction (both diastolic and systolic), and atrial fibrillation (AF) and dilatation. Moreover, LVH reduces the coronary reserve, increasing the risk of ischemic heart disease.51 Controlling ventricular remodeling as far as possible has thus become one of the therapeutic targets in the management of chronic diseases in which LVH is prevalent, such as diabetes mellitus and chronic renal failure.
Diastolic Ventricular DysfunctionDuring diastole, the LV receives blood from the left atrium, which is subsequently ejected into the systemic circulation. In simple terms, the efficiency of LV filling can be measured as the ability to receive a large volume of blood at rapid filling rate but under low pressures.52 Consequently, various physiological parameters interact in LV diastole, principally relaxation, ventricular distensibility and atrial contraction (Table 2). Among the most common causes of diastolic dysfunction are PH, senility and CAD.
Application of Doppler in the Diagnosis of Diastolic Dysfunction and Parameters Observed in the Slow Relaxation Pattern.
E/A: ratio between the early ventricular filling wave and atrial contraction; EDT: deceleration time of the early ventricular filling wave; Em: diastolic velocity of the myocardium obtained by tissue Doppler during early filling; Vp: color M-mode flow propagation velocity slope.
Adapted from García et al.40
Various studies in COPD patients describe a high rate of LV diastolic dysfunction (LVDD) compared to age-matched controls, even in those without CVRF.53–70 Its prevalence also varies considerably, reaching 90% in COPD patients with severe airflow limitation.68 The most frequently described pattern is slow relaxation, which is characterized by a reduced E wave (due to a decrease in the relaxation velocity of the myocardial fibers) and an increased A (atrial contraction) wave with an E:A ratio <1.40 This impaired ventricular filling can be a major complication in patients with AF, a very prevalent arrhythmia in COPD, due to both loss of atrial systole and shortening of the filling period.59
Several authors attributed this impairment to the phenomenon of ventricular interdependence. Funk et al.,59 however, described LVDD in COPD patients without PH, demonstrating that its development may be due to the interaction of other mechanisms, as described above.
Impaired ventricular filling has been negatively associated with exercise tolerance assessed using the 6-min walk test,11,68 and with a reduction in physical activity.71
Bhattacharyya et al.65 conducted a study to determine whether LVDD could be secondary to ischemic lesions that were not detected by conventional studies (electrocardiogram and echocardiography). They included patients with COPD GOLD III and IV with no risk factors for developing LVDD (HT, diabetes mellitus, history of ischemic heart disease or hypothyroidism). Patients diagnosed with LVDD by echocardiography were examined using myocardial perfusion single-photon emission computed tomography imaging. Reversible perfusion defects were found in 7 of the 14 patients studied (50%). On the basis of these findings, the authors hypothesized that ischemic heart disease in these patients could also be a cause of LVDD. Further studies are required with a larger patient sample to confirm these results.
Systolic Ventricular DysfunctionLeft ventricular systolic dysfunction (LVSD) is defined as an LVEF <50%.72 The reported prevalence of LVSD in patients with stable COPD varies widely, and is related to the exclusion of CVRF in different series (0%–16% in COPD patients without CVRF).73 The frequency of this abnormality ranges from 8% to 25% in non-selected patients.62,63,66,67
Analysis of ventricular deformation or strain and the ventricular deformation rate or strain rate are the new parameters that quantitatively assess the segmental contractility of the LV, irrespective of a normal LVEF. This can be done using tissue Doppler imaging, and more recently, using two-dimensional ultrasound speckle tracking. Sabit et al.61 found that these parameters were significantly reduced compared to controls in a series of 36 patients with COPD. Schoos et al.,70 meanwhile, reported that these parameters were predictors of mortality by multivariate analysis in a series of 90 patients with COPD of varying severity.
LVD (systolic and diastolic) was described as a predictor of survival in a cohort of COPD patients undergoing vascular surgery who were followed up for 2 years.62 Macchia et al.66 found higher mortality in COPD patients with this abnormality compared non-LVD patients, although this difference did not reach statistical significance.
Heart FailureHeart failure (HF) may not be suspected in patients with COPD, since the signs and symptoms are common and overlap in both diseases. The prevalence of HF in patients with COPD in different series ranges from 7.1% to 31.3%.4,32–35,62,74
There are 2 patterns of HF, one involving preserved systolic function (HFpEF), more associated with HT, advanced age and extracardiac diseases, and the other involving a reduced ejection fraction (HFrEF), more related with ischemic heart disease.
Rutten et al.75 reported a prevalence of HF of 20.5% in a cohort of patients with stable COPD. During a 4-year follow-up period, mortality among patients diagnosed with HF was higher, regardless of other factors such as age, sex, history of ischemic heart disease or HT.76 Although there is little information in this respect, the prognosis in COPD patients with HF appears to be independent of LVEF.77
Conversely, COPD is a common comorbidity in patients with HF, and has been described in up to one third of this population.78 Comorbid COPD has been shown to worsen the prognosis in HF, and COPD exacerbation is known to cause or precipitate acute HF.72 In a study comparing the impact of different comorbidities on prognosis in 2843 patients diagnosed with HFpEF and 6599 with HFrEF, COPD was the only comorbidity that acted as an independent variable of mortality for both groups.79 It is remarkable that, despite the importance of COPD as a comorbidity in HF, the latest HF guidelines do not include spirometry among the complementary tests recommended in the management of this entity.
ConclusionsLV defects can present in patients with COPD of any severity. The pathophysiological mechanisms involved can act independently or synergically, given the complex heart–lung interaction. While little information is currently available, studies have shown that LV defects in COPD negatively affect parameters as important as exercise capacity, physical activity and mortality. These findings pave the way for future research, and to new diagnostic and therapeutic strategies. However, it should be borne in mind that treatment and prevention of smoking has the greatest impact on the natural history of coexisting COPD and CVD. In view of recent research, it is essential that specialists in other fields contribute to the study of this association to find a comprehensive approach to the disease, in the certainty that COPD does not live (or die) by cor pulmonale alone.
Conflict of InterestsNone declared.
We would like to thank Dr. Ignasi Guasch for the radiological imaging.
Please cite this article as: Portillo K, Abad-Capa J, Ruiz-Manzano J. Enfermedad pulmonar obstructiva crónica y ventrículo izquierdo. Arch Bronconeumol. 2015;51:227–234.