Since the publication, 9 years ago, of the latest SEPAR (Spanish Society of Pulmonology and Thoracic Surgery) Guidelines on Difficult-to-Control Asthma (DCA), much progress has been made in the understanding of asthmatic disease. These new data need to be reviewed, analyzed and incorporated into the guidelines according to their level of evidence and recommendation. Recently, consensus documents and clinical practice guidelines (CPG) addressing this issue have been published. In these guidelines, specific mention will be made of what the previous DCA guidelines defined as “true difficult-to-control asthma”. This is asthma that remains uncontrolled after diagnosis and a systematic evaluation to rule out factors unrelated to the disease itself that lead to poor control (“false difficult-to-control asthma”), and despite an appropriate treatment strategy (Spanish Guidelines for the Management of Asthma [GEMA] steps 5 and 6): severe uncontrolled asthma. In this respect, the guidelines propose a revised definition, an attempt to classify the various manifestations of this type of asthma, a proposal for a stepwise diagnostic procedure, and phenotype-targeted treatment. A specific section has also been included on DCA in childhood, aimed at assisting healthcare professionals to improve the care of these patients.
Desde la publicación, hace ya 9 años, de la última normativa de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) sobre asma de control difícil (ACD), se han producido avances en los conocimientos de la enfermedad asmática, que hacen necesario realizar una puesta al día de los datos disponibles e incorporarlos tras su análisis en el nivel de evidencia y recomendación más adecuado. Recientemente han aparecido documentos de consenso y guías de práctica clínica (GPC) que abordan este problema. En esta normativa se hará mención explícita a lo que la previa guía de ACD definía como «verdadera asma de control difícil»; es decir, al asma que tras haber verificado su diagnóstico, realizado un abordaje sistematizado para descartar factores ajenos a la propia enfermedad que conducen a un mal control de la misma («falsa asma de control difícil»), y realizar una estrategia de tratamiento adecuado (escalones 5 y 6 de la Guía española para el manejo del asma [GEMA]), no se consigue alcanzar el control: «asma grave no controlada» (AGNC). En esta línea la normativa propone una revisión de la definición, un intento de clasificación de las diferentes manifestaciones de este tipo de asma, una propuesta del abordaje diagnóstico por pasos y un tratamiento dirigido según fenotipo, conjuntamente con un apartado específico sobre este arquetipo de asma en la infancia, con el objetivo de que pueda servir de ayuda a los profesionales sanitarios y repercutir en el cuidado de estos pacientes.
Asthma is a worldwide problem, and severe uncontrolled asthma (SUCA) in particular has far-reaching socioeconomic repercussions. For this reason, all healthcare professionals treating these patients must be aware of the situation, and have the proper tools for providing the best approach to this problem.
The first obstacle encountered is the exact terminology for defining these patients, since there is no common agreement.
“Difficult-to-treat asthma” is a term used for patients who find it difficult to achieve control as a result of poor compliance, incorrect inhalation techniques, exposure to allergens or other triggers, or associated comorbidities. The term “refractory or treatment-resistant asthma” refers to subjects who, when a diagnosis of asthma has been confirmed and comorbidities identified and treated, require treatment with high-dose inhaled corticosteroids (IC) plus another second controller (long-acting β2-agonist [LABA] and/or systemic corticosteroids [SC]) to prevent their asthma becoming uncontrolled, or who remain uncontrolled despite this therapy. SUCA includes patients with refractory asthma and those with incomplete response to treatment.1
MethodologyThese guidelines were drawn up following SEPAR recommendations for the development of guidelines. All citations used by the various authors, experts in severe asthma, were recorded in an EndNote database and classified according to their levels of evidence, using the Grading of Recommendations (GRADE) approach.2
Among the studies selected from a systematic search as a basis for these guidelines, very few were identified as randomized controlled trials with a low risk of bias that would provide direct, consistent and precise evidence. Most recommendations proposed are based on indirect evidence, retrieved from studies performed in moderate persistent asthma, and to a lesser extent, studies in patients with severe asthma. The result is imprecise levels of evidence and recommendations, although all the studies were evaluated and classified using the GRADE system.
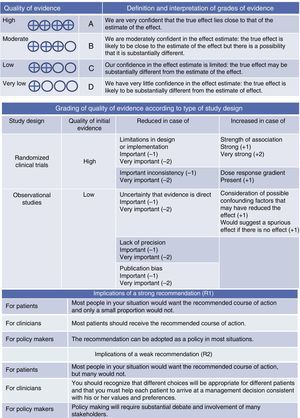
Quality of evidence was classified as high, moderate, low and very low, based on different considerations for presence of direct bias (and the direction), consistency and directness of the estimates (Fig. 1).
DefinitionThe definition set out in the ATS/ERS consensus statement1 was used throughout this paper. This defines severe asthma as “asthma requiring treatment with high-dose IC, plus a second controller and/or SC to prevent it from becoming uncontrolled or that remains uncontrolled despite this therapy” (evidence D-R2). Severe uncontrolled asthma is defined in Table 1.
Definition of Severe Asthma in Patients Older Than 6 years.
| The ATS/ERS Consensus defines severe asthma as asthma requiring treatment at steps 4–5 of the Global Initiative for Asthma (GINA) clinical practice guidelines, namely high-dose inhaled corticosteroids and a long-acting beta-antagonist, or a leukotriene modifier, or theophylline use in the previous year, or systemic corticosteroids for ≥50% of the last year, to prevent the disease from becoming uncontrolled, or disease that remains uncontrolled despite this treatment |
| The Consensus defines uncontrolled asthma as at least one of the following: |
| Poor control of symptoms measured on questionnaires evaluating disease control, such as the ACT and ACQ. ACQ scores >1.5 or ACT ≤19 are considered criteria for poor control according to clinical practice guidelines, such as GINA/National Asthma Education and Prevention Program (NAEPP) |
| Frequent severe exacerbations: 2 or more exacerbations requiring bursts of systemic corticosteroids for 3 or more days each in the previous year. |
| Exacerbations requiring at least 1 hospitalization, ICU admission or need for non-invasive mechanical ventilation in the previous year. |
| Airflow limitation: FEV1 remains <80% predicted value after use of an appropriate bronchodilator and FEV1/FVC ratio below the lower limit of normal (when best FEV1 is >80%). |
| Controlled asthma that worsens after reducing high-dose inhaled or systemic corticosteroids or new biologics |
| Inhaled Corticosteroid | Threshold Dose (μg) Considered High | |
|---|---|---|
| Age 6–12 years | Age >12 years | |
| Beclomethasone dipropionate | ≥800 (DPI or CFC MDI) | ≥2000 (DPI or CFC MDI) |
| ≥320 (HFA MDI) | ≥1000 (HFA MDI) | |
| Budesonide | ≥800 (MDI or DPI) | ≥1600 (MDI or DPI) |
| Ciclesonide | ≥160 (HFA MDI) | ≥320 (HFA MDI) |
| Fluticasone dipropionate | ≥500 (HFA MDI or DPI) | ≥1000 (HFA MDI or DPI) |
| Momethasone furoate | ≥500 (DPI) | ≥1000 (DPI) |
| Triamcinolone acetonide | ≥1200 | ≥2000 |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; CFC: chlorofluorocarbons; DPI: dry powder inhaler; FEV1: forced expired volume in 1s; FVC: forced vital capacity; GINA: Global Initiative for Asthma; HFA: hydrofluoroalkanes; MDI: measured dose inhaler; NAEPP: National Asthma Education and Prevention Program; ICU: intensive care unit.
Adapted from ATS/ERS.1
The lack of well-designed studies using homogeneous definitions means that available data on the epidemiology of severe asthma are disparate, particularly in adults. Severe asthma varies in prevalence between one country and another (18% in Western Europe, 19% in the United States and 32% in Central Europe)3 and about 50% of these severe patients are thought to have poor disease control.3 In Spain in 2011, the prevalence of severe uncontrolled asthma, according to medical criteria, was reported to be 3.9% of all asthma cases4 (evidence C). Moreover, this small proportion of patients is responsible for much higher use of resources than other asthmatic patients5 (evidence D-R2).
GeneticsAsthma is a complex syndrome resulting from the interaction of numerous genes and environmental exposure. The lack of studies in severe asthma make it impossible to determine precisely which genes are responsible for making an individual susceptible to developing severe asthma. Currently, genome wide association approaches (GWAS) are analyzing hundreds of thousands of polymorphisms located throughout the genome in the search for variants associated with susceptibility to developing severe asthma6 (evidence C-R2). Single nucleotide polymorphisms (SNPs) in the interleukin-4 receptor subunit alpha (IL4Rα) are associated with poorer lung function, higher immunoglobulin E (IgE) levels, severe asthma exacerbations and tissue inflammation7 (evidence C-R2). An IL-6 receptor (IL6R) variant is associated with severe asthma phenotypes and poorer lung function8 (evidence C-R2). Other genetic mutations associated with severe asthma are variations in genes involved in tumor necrosis factor (TNF) and multiple SNPs in the RAD50-IL13 regions of chromosome 5q31.1 and the HLA-DR/DQ region of chromosome 6p21.3, respectively9 (evidence C-R2).
Severe Asthma PhenotypesClinicians have identified markedly different asthma patient subtypes or profiles, suggesting that asthma remains a poorly classified syndrome.
Several studies that merge objective clinical variables have been performed in an attempt to categorize patients into clinical phenotypes, or on their pathophysiological basis (endotypes)10 which, in the case of SUCA, will have therapeutic implications (evidence D-R2).
These studies share several multivariate statistical techniques, especially cluster analyses11–13 (evidence C-R2). The overall conclusion is that there are at least 4 reasonably well defined phenotypes/endotypes in severe asthma, characterized according to natural history, pathobiology, clinical features and therapeutic response.
Severe Allergic AsthmaAbout 40%–50% of cases of severe asthma are due to allergies. They begin in infancy, are clearly atopic and appear to progress from mild-moderate asthma, although some patients present the severe form from onset. The most severe cases within this cluster are those with a greater number of positive skin pricks or higher IgE levels in blood, those with a family history of asthma, and a longer time since disease onset. However, not all patients have eosinophil predominance; indeed, the most severe forms of the phenotype (in terms of lung function, frequency and intensity of symptoms and use of healthcare resources) are accompanied by increases in both eosinophils and neutrophils in sputum14 (evidence C-R2).
From a pathobiological point of view, severe allergic asthma is orchestrated by the activation of type 2 helper T-cells (Th2), the production of specific cytokines, IL-4, IL-5 and IL-13, and B-cell isotype switching to produce IgE, although it must be pointed out that not all atopic asthmas have a highly active Th2 component (“high” Th2 endotype). Periostine and fractionated exhaled nitric oxide (FeNO) have been shown to be good biomarkers in this “high” Th2 variant15 (evidence C-R2).
Late Onset Eosinophilic AsthmaOver 25% of cases of severe asthma are thought to belong to this phenotype. They are characterized by persistent eosinophils in bronchial biopsies and induced sputum, despite high-dose IC or SC. Levels of eosinophils and cysteinyl leukotrienes are higher than in severe allergic asthma. In general, the disease occurs in patients older than 20 years, and may be preceded by symptoms of chronic rhinosinusitis and nasal polyps. A subgroup of these patients also develops intolerance to non-steroidal anti-inflammatory drugs (NSAIDs), designated as “aspirin-exacerbated respiratory disease” (AERD). Clinical symptoms are florid from onset, airway obstruction is considerable and exacerbations are frequent. These patients are generally less atopic than those in whom the disease appears at younger ages and they have little family history of asthma, but their IgE levels and FeNO measurements may be equally high. Its pathogenesis is related to changes in arachidonic acid metabolism (decreased production of prostaglandin E2 and increased cysteinyl leukotriene synthesis), associated with inflammation that appears to related with Th2 activation.15
Severe Non-Atopic Asthma in the Obese AdultThis population mainly comprises women with a high body mass index and abundant clinical signs and symptoms. Onset occurs between 50 and 60 years of age, or even later. Sputum eosinophilia is not always detected; exacerbations are frequent and lung function is not overly compromised. The genetic basis and etiology of the disease are not well understood, nor is the role of hormonal factors, although onset after natural or surgical menopause has often been reported. The association between asthma and obesity raises numerous questions, and a number of mechanisms have been proposed as its cause: immunoinflammatory factors, mechanical factors, loss of corticosteroid efficacy, vitamin D deficiency, and the additional burden of other comorbidities, such as obstructive sleep apnea–hypopnea syndrome16 (evidence D-R2).
Adult-Onset Neutrophilic AsthmaThe natural history of adult-onset neutrophilic asthma is not well understood. Increased matrix metalloproteinase-9 levels have been found in bronchoalveolar lavage, and a history of smoking and chronic airflow limitation (CAFL) with significant air trapping have also been observed. SC provide little disease control. Neutrophil predominance in the airways may be due to modifications in the expression of genes responsible for their activation and mobilization, concomitant diseases (bronchiolitis obliterans), or residual airway inflammation after continuous use of SC, inhibiting neutrophil apoptosis.15
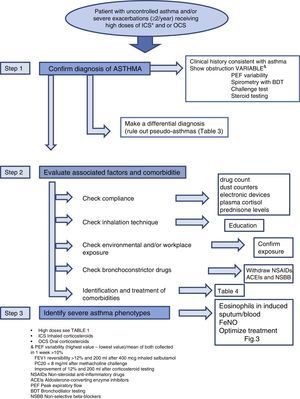
Diagnosis of the Patient With Severe Uncontrolled AsthmaDiagnosis of SUCA requires systematic stepwise assessment to ensure that no relevant areas are left unexplored. According to the previous SEPAR guidelines17 (evidence D-R2) and the recommendations of the current consensus statements1,18,19 (evidence D-R2), this assessment must be performed in accredited units or in reference centers, by professionals with documented clinical experience in the management of these patients, in follow-up visits over a period of not less than 6 months (Fig. 2).
Step 1: Determine if the Patient has AsthmaA diagnosis reached in the past by other clinicians and provided by the patient must not be accepted unless the objective test results necessary for confirming the diagnosis are available. This attitude has been supported in several studies which found that a previous diagnosis of asthma was overturned in almost one third of patients after a new systematic assessment20 (evidence D-R2).
Clinical History and Tests to Confirm AsthmaRe-assessment is recommended, with a full clinical history investigating the onset of symptoms, their variability over time, and their association with specific triggers that would raise the suspicion of asthma or any other confounding disease. Variable airflow obstruction should be confirmed with standard lung function testing (LFT), according to the recently revised criteria in the latest GINA update21 (evidence D-R2GPC).
Differential DiagnosisCorrect differential diagnosis (DD) should consider other diseases that might mimic asthma, but are not true asthma, also known as “pseudo-asthmas or false asthmas”. Diseases that can mimic asthma are listed in Table 2.
Differential Diagnosis of Asthma in Adults and Children.
| Adults | Children |
|---|---|
| Upper airway diseasesOrganic causesTumors of the tracheaCongenital airway abnormalitiesCongenital vascular abnormalitiesTracheobronchomalaciaIdiopathic relapsing polychondritisTracheal amyloidosisIBD-associated airway stenosisEndothoracic goiterChronic fibrosing mediastinitisFunctional causesVocal cord dysfunctionLower airway diseasesChronic obstructive pulmonary diseaseBronchiolitis obliteransFunctional or psychogenic dyspneaOther organ diseaseLeft heart failure | Upper airway diseasesAdenotonsillar hypertrophyLaryngomalacia, vocal cord paralysis, laryngeal membraneVocal cord dysfunctionLarge airway obstructionTracheomalacia, bronchomalaciaTracheal or bronchial stenosisForeign bodyVascular ringsLymphadenopathies, tumorsSuppurative lung diseaseCystic fibrosisBronchiectasisPrimary ciliary dyskinesiaPersistent bacterial bronchitisImmune deficienciesAspirative syndromesGastroesophageal refluxUncoordinated swallowingTracheoesophageal fistulaLaryngeal cleftOtherBronchopulmonary dysplasiaBronchiolitis obliteransPulmonary edemaPsychogenic cough |
IBD: inflammatory bowel disease.
False asthmas are often diagnosed months or even years after the onset of clinical symptoms. This delay in diagnosis often leads to excessive use of anti-asthmatic medications, including SC, that cause major side effects (osteoporosis, glaucoma, obesity, hypertension). These patients also repeatedly attend the emergency department, where they are given high dose combinations of anti-asthma drugs, even if these are obviously ineffective in controlling the disease.18
The diagnostic tests to be performed in DD are shown in Table 3.
Differential Diagnosis of Severe, Uncontrolled Asthma in Adults. Pseudo-Asthmas and Diagnostic Tests.
| Upper airway organic diseaseBronchial obstruction | Spirometry with flow-volume loopUpper airway inspiratory/expiratory CTFiberoptic bronchoscopy |
| Vocal cord dysfunction | Laryngoscopy during attack or after methacholine challenge or after ergometry |
| Chronic obstructive pulmonary disease (emphysema) | Chest HRCTPlethysmography and diffusion |
| Bronchiolitis obliterans | Inspiratory/expiratory chest HRCTPlethysmography/air trappingTransbronchial/pulmonary biopsy |
| Functional dyspnea | Nijmegen hyperventilation questionnairePsychological assessment |
| Left heart failure | Chest HRCTECG/echocardiogram |
| BronchiectasisCystic fibrosisABPA | Chest HRCTSweat testTotal and Aspergillus specific IgE/precipitin |
| Churg-Strauss syndromePulmonary eosinophilia | pANCA/biopsy of affected organ(s)Fiberoptic bronchoscopy (BAL) |
ABPA: allergic bronchopulmonary aspergillosis; pANCA: perinuclear anti-neutrophil cytoplasmic antibodies; ECG: electrocardiogram; IgE: immunoglobulin E; BAL: bronchoalveolar lavage; CT: computed tomography. HRCT: high-resolution CT.
In this group, diseases with an organic origin must be distinguished from functional conditions.
Organic CausesStridor suggests that the obstruction is located in the upper airways, perhaps due to altered morphology (flattening) of the flow-volume loops. If this is suspected in an asthma patient who does not respond to treatment, end-inspiratory and expiratory computed tomography (CT) is justified to quantify the degree of tracheo- and bronchomalacia. Another alternative is fiberoptic bronchoscopy.
Functional CausesSome individuals, for reasons unknown, have vocal cords that remain partially closed during inhalation, causing a sensation of dyspnea in the form of acute asphyxia, often accompanied by stridor. Vocal cord dysfunction can occur without any apparent triggering factor, or as a reaction to various stimuli, such as physical exercise, as is often seen in athletes22 (evidence D-R2). It occurs in children and adults and is more common in women and subjects with high scores on anxiety questionnaires. Despite the apparent severity of some episodes, it rarely leads to loss of consciousness and, in the vast majority of cases, abates in minutes. Up to 50% of patients referred to a DCA specialist clinic have vocal cord dysfunction23 (evidence C-R2). Ideally, the diagnosis is confirmed from poor functioning of the cords, when an episode coincides with laryngoscopy or video laryngo-stroboscopy. As this is not always possible, the event can be induced by methacholine challenge testing. If dysfunction occurs during exercise, it can be reproduced in the laboratory by means of an exercise tolerance test.22
These patients are often subjected to unnecessary endotracheal intubation: if there is no resistance to ventilation after tube placement, the patient can be extubated after a few minutes and recovery is rapid.
Lower Airway Diseases. Chronic obstructive pulmonary disease (COPD) is a condition that presents with exacerbations that mimic persistent asthma with a partially reversible obstructive component. An asthmatic smoker can simultaneously develop COPD; this presentation is known as asthma/COPD overlap syndrome (ACOS).21 Clinical history, radiological examinations, and LFT can help differentiate between asthma and COPD. In these cases, lesions indicative of emphysema, absent in asthma, may be seen on CT. LFT may show marked reversibility, associated with other findings more typical of COPD/emphysema, such as air trapping or reduced carbon monoxide diffusion capacity (DLCO). SC testing may also be performed.18
Lower airway compromise due to other diseases, such as bronchiolitis obliterans, should be suspected in patients with a mosaic pattern on CT or signs suggestive of hyperinflation on LFT. Lung biopsy should be considered for confirming diagnosis in these patients.
Functional Dyspnea. Dyspnea is a subjective sensation of breathlessness that can be described by the patient in a variety of ways: difficulty inhaling, difficulty exhaling, chest tightness. Dyspnea not associated with any organic disease is called functional dyspnea or breathing dysfunction24 (evidence C-R2).
This entity can vary widely in intensity, from a feeling of moderate difficulty in filling the lungs to acute hyperventilation attacks. About 10%–20% of the general population suffer sighing dyspnea, but for unknown reasons, this figure rises to 30%–40% in asthma sufferers. Functional dyspnea is more common in women, particularly those with high scores on anxiety questionnaires.24 When sighing dyspnea occurs along with asthma, severity is often misjudged, and the condition is over-treated. To distinguish between dyspnea caused by asthma or a functional disorder, objective data on the degree of lung function compromise must be collected and peak expiratory flow must be determined to help correlate symptoms and changes in lung function. Functional dyspnea and vocal cord dysfunction may occur concomitantly in some patients.
Diseases Affecting Other Organs. Left heart failure may cause apparent worsening of asthma. Onset of atrial fibrillation or mildly decompensated hypertensive heart disease or coronary artery disease can turn well-controlled bronchial asthma into a treatment-refractory problem. In these cases, correcting the arrhythmia or heart failure can lead to stabilization of the asthma.
Step 2: Identify Factors for Poor Control and Evaluate Concomitant DiseasesCheck ComplianceNumerous studies have confirmed that lack of treatment compliance or poor adherence is more common than thought. Up to 46% of asthma patients fail to comply adequately with their prescribed treatment25 (evidence C-R2).
Measuring treatment compliance is a complex task in any chronic disease, and perhaps even more so when adherence to an inhaled medication is being evaluated. In practice, compliance is determined according to clinical judgment, therapeutic response or validated questionnaires, although these methods are all known to overestimate the level of treatment adherence. FeNO has been proposed for measuring compliance, given the good response of this marker to inhaled corticosteroids, and various procedures for determining this parameter have been described (Fig. 2).
Check Inhalation TechniqueErrors in inhalation technique are much more common than might be imagined. A recent study26 (evidence D-R2) found that critical errors were being made by 12% of metered dose inhaler (MDI) users, 35% of Diskus® and HandiHaler® users and 44% of Turbuhaler® users. Incorrect inhaler use was associated with increased hospital admission, visits to emergency departments, SC treatment and antibiotics, and worse scores in the Asthma Control Test (ACT).
In this respect, GEMA recommends the use of MDI with a chamber to improve the distribution and amount of drug reaching the bronchial Tree27 (evidence D-R2GPC).
Inhalation technique training and verification is a necessary step in the education of asthma patients. The inhalation device must be selected on the basis of various considerations: patient preference, peak inspiratory flow, device resistance, and local adverse effects.
Check Environmental and/or Workplace ExposureNo established consensus statement is available for identifying exposure to environmental triggers, even if these are known to play an important role in patients with severe asthma. The role of exposure to agents in the workplace must also be taken into account (over 200 have been identified), particularly in adult-onset asthma. Exposure to allergens known to trigger allergies should be avoided, although the evidence remains insufficient.21
Check Bronchoconstrictor DrugsIn some patients, uncontrolled asthma may be due to the use of medications (NSAIDs, angiotensin-converting enzyme inhibitors [ACEIs], non-selective beta-blockers) that can cause persistent asthma symptoms.18
Evaluate ComorbiditiesSome conditions or diseases occur more commonly in asthma patients than in the general population (Table 4). While these relationships are known to contribute to insufficient asthma control, they have not been precisely identified, and still need to be fully characterized.
Evaluation and Treatment of Comorbidities.
| Comorbidity | Diagnostic tests | Treatment |
|---|---|---|
| Rhinosinusitis | RhinoendoscopyCT of paranasal sinuses | AntileukotrienesIntranasal corticosteroidsNasal saline washingNasal endoscopic surgery |
| Gastroesophageal reflux | Esophageal pH testing/manometryTherapeutic trial with PPI | Health and dietary advicePPISurgery |
| Obesity | BMI | Weight lossBariatric surgery |
| OSAHS | Polysomnography | CPAPWeight loss, if applicable |
| Psychiatric disease | Assessment by psychologist/psychiatrist | PsychotherapySpecific treatment |
| Functional dyspnea | Specific questionnaires(Nijmegen) | PsychotherapyRespiratory re-education |
| Vocal cord dysfunction | Laryngoscopy during attack or methacholine challenge/exercise test | Speech therapy rehabilitation |
CPAP: continuous positive airway pressure; PPI: proton pump inhibitors; BMI: body mass index: OSAHS: obstructive sleep apnea–hypopnea syndrome; CT: computed tomography.
Although the impact of treatment of comorbidities on severe asthma remains to be determined, they should be treated as appropriate to improve them as far as possible.1
Step 3: Identify Severe Asthma PhenotypesTo date, there is no generally accepted definition of what has become known as asthma phenotypes, but there does seem to be consensus with regard to certain clinical profiles of patients with distinct pathophysiological characteristics that cause them to respond better to certain medications. Attempting to define these phenotypes may be of use in optimizing treatment, and to this end, certain clinical characteristics may be of interest, e.g., age of onset, obesity, NSAID intolerance, in addition to laboratory parameters, such as eosinophils in induced sputum and blood, FeNO determinations, and skin prick tests (Fig. 2).
Treatment of Severe Uncontrolled AsthmaUntil recently, few clinical trials were specifically designed for evaluating the efficacy of different treatments in the SUCA population. Now, however, the identification of new therapeutic targets has led to the development of studies with some scientific evidence of efficacy in these patients, albeit with short follow-up.
Corticosteroid InsensitivityAlthough SC are very effective in controlling inflammation in asthma, the spectrum of response to these agents among asthmatics is broad, and a small percentage of subjects fail to respond at all, despite the administration of high doses. Total resistance to SC is uncommon; partial resistance or “lack of sensitivity” is more common among severe asthmatics, making high SC doses necessary, although not even with these is full control achieved. Some patients do improve notably with the use of SC, but when they are discontinued, the asthma worsens (corticosteroid-dependent asthma). In a study of 102 SUCA children, only 11% did not respond to an intramuscular (IM) dose of triamcinolone, suggesting that 89% had some degree of response to SC28 (evidence D-R2). Accordingly, the recent ATS/ERS consensus1 uses the expression “SC insensitivity” instead of “resistance”, and we have adhered to this nomenclature in these guidelines.
From a clinical point of view, SC-insensitive asthma is defined by forced expiratory volume in the first second (FEV1) less than 75% of the predicted value and a response <15% and 200ml after the administration of a 2-week cycle of 40mg/day prednisone or prednisolone29 (evidence D-R2). These patients have normal plasma cortisol and adrenal suppression response to exogenous cortisol, so they are prone to the side effects of these agents. Factors contributing to asthma insensitivity to SC are not well defined. Some studies report weak associations between certain genetic changes and environmental factors (continuous exposure to allergens, smoking, NSAID intolerance, low vitamin D levels, and chlamydia, mycoplasma or viral infections), but these cannot be considered as clearly established risk factors. The most significant environmental factor in SC insensitivity is exposure to tobacco smoke. This may act by various mechanisms: by altering the inflammatory pattern (increased neutrophils and CD8 lymphocytes and reduced eosinophil levels), by impairing mucociliary function, thus allowing excessive mucous deposition in the airways, or by tobacco-induced oxidative stress, which inactivates histone deacetylase, reducing nuclear translocation and the number of corticosteroid receptors and their affinity30 (evidence D-R2).
SC act at different levels, but their greatest anti-inflammatory effect is produced by inhibition of the genetic transcription of numerous genes that code for proinflammatory proteins (cytokines, chemokines, adhesion molecules) and by increased transcription of anti-inflammatory mediators. They act by binding to a specific intracytoplasmic receptor with 2 molecular isoforms (α and β). The β receptor cannot bind to the hormone, and, as such, is inactive31 (evidence D-R2). Lack of sensitivity to SC cannot be explained by pharmacokinetic changes or malabsorption, and is probably due to a range of different mechanisms32 (evidence C-R2) (Table 5).
Glucocorticoid Resistance Mechanisms in Severe Asthma.
| Reduced number of GC receptors |
| Increased GC β-receptor expression |
| Cytokines inducing suppression of GC activity |
| Alteration in the GC receptor affinity |
| Reduced GC-receptor nuclear translocation |
| Reduced histone acetylation and increased activation of the protein-1 (AP-1) pathway and nuclear factor κB (NF-κB) |
| Predominantly neutrophil inflammation |
GC: glucocorticoids.
Taken from Reddy and Little.32
Response to IC varies notably among individuals11 (evidence C-R2). However, there is some evidence to suggest that SUCA patients may respond to higher doses than normally recommended33 (evidence A-R1). Although some studies support the greater therapeutic efficacy of fine particle IC (related with their effect on the peripheral airways), there is no evidence of their superiority in SUCA34 (evidence C-R2).
Among the new glucocorticoids, ciclesonide has fewer local and systemic side effects since it is a prodrug converted to its active form in the lung parenchyma35 (evidence B-R2). New steroids, known as “dissociated” compounds (mapracorat), that aim to separate the anti-inflammatory mechanisms from the side effects, are currently under development36 (evidence D-R2).
Systemic CorticosteroidsThe best time for introducing maintenance treatment with SC is not well defined, nor is there evidence that continuous treatment with low-dose SC is more effective than cycles of SC in reducing the number of exacerbations. IM triamcinolone administration (Trigon® depot 40mg) in asthmatics with corticosteroid insensitivity improves control, reduces eosinophils in sputum, increases FEV1 and prevents exacerbations. Reasons for its efficacy may include reinforced compliance or the higher dosage of triamcinolone compared to other corticosteroids used in the clinic37 (C-R2).
Long-Acting β2-Adrenergic AgonistsAdding a LABA to an IC has been shown to be more effective than doubling the dose of the IC or adding an antileukotriene, although there may be notable variability in response, which needs to be monitored38 (evidence A-R1). Formoterol as a complete agonist has greater intrinsic efficacy and causes a greater number of adverse effects39 (evidence D-R2), the most common of which are tachycardia and hypokalemia, which can be more pronounced in individuals homozygous for arginine in position 16 of β2-AR.
Long-Acting AnticholinergicsRecent studies have shown that long-acting anticholinergics (LAMA) may be useful in patients with severe asthma and concomitant CAFL40 (evidence B-R2), in cases of ACOS, in severe asthma with a non-eosinophilic inflammatory profile41 (evidence D-R2), and in asthmatics with the ArgGly variant in codon 16 of the β2-receptor42 (evidence B-R2). There is an increasing trend in the use of LAMA as a treatment for bronchial asthma, and some CPG suggest their use in the higher stages of severity, when control cannot be achieved.
Vitamin DLevels of 25-hydroxyvitamin D<30ng/ml are related with asthma severity, increased risk of exacerbations and poor lung function, and are also implicated in the mechanisms of steroid insensitivity. However, no definitive results are available to support supplementation with this vitamin43 (evidence D-R2).
MacrolidesTreatment with macrolides for 3 weeks or more in severe asthma is not associated with a significant improvement in FEV1, although improvement has been reported in morning PEF, symptom control, bronchial hyperreactivity, and health-related quality of life (HRQoL)44 (evidence D-R2). There is insufficient evidence on generalized use for reducing the number of exacerbations, although some studies recommend macrolides in the severe neutrophilic asthma phenotype45 (evidence B-R2).
AntileukotrienesPatients with AERD generally have excessive basal leukotriene production, so would appear to be ideal candidates for antileukotriene treatment46 (evidence C-R2). Some recent studies report findings in air trapping and radiological changes on CT that suggest montelukast may be useful, in general, in patients with severe asthma, so it may tried as an add-on therapy in these patients47 (evidence C-R2).
OmalizumabAnti-IgE treatment has shown clinical efficacy in inflammatory response markers, frequency of exacerbations, visits to emergency departments, severity of symptoms, use of IC, and HRQoL. Omalizumab is indicated for improving asthma control when administered as add-on treatment in adults and older children (over 6 years of age) with persistent severe uncontrolled allergic asthma with perennial allergies, reduced lung function and documented severe exacerbations, despite appropriate treatment for their level of severity48 (evidence B-R2). Data from non-atopic patients receiving omalizumab have been published, which could open a new avenue for treatment in this patient group49 (evidence D-R2).
ThermoplastyAlthough early clinical studies performed in asthmatics classified as moderate and severe have not shown particularly favorable results, due to adverse events and the lack of effect on bronchial hyperreactivity50 (evidence B-R2), it is not yet clear which subgroup of patients might benefit from this technique. A recent study, with a 5-year follow-up, showed a sustained improvement in disease control (reduction in number of severe exacerbations and visits to the emergency room) and confirmed the safety of the procedure51 (evidence B-R2). It is currently recommended only in experienced units and in the research setting1,52 (evidence D-R2)53 (evidence B-R2).
Theophylline and Phosphodiesterase-4 InhibitorsAlthough no controlled trials have been performed in severe asthma, theophylline as a single agent has relatively weak anti-inflammatory activity, but at low doses it can markedly enhance the action of corticosteroids on the expression of inflammatory genes54 (evidence D-R2).
Data are also available on the utility of the new phosphodiesterase-4 inhibitors (roflumilast) in asthmatics55 (evidence B-R2) and the 2014 Spanish COPD Guidelines (GesEPOC) update continues to suggest these drugs as add-on treatment in ACOS patients with uncontrolled symptoms56 (evidence D-R2CPG).
Other TreatmentsStudies have confirmed the unfavorable risk/benefit profile of etanercept, methotrexate and cyclosporin.
An anti-IL5 monoclonal antibody, mepolizumab, has been recently investigated in patients with eosinophilic asthma, showing significant improvements in HRQoL, exacerbations and symptoms57 (evidence A-R1)58 (evidence B-R2) and reduced use of SC59 (evidence B-R2). Studies with reslizumab and benralizumab (anti-IL-5 receptor) are opening up new prospects60 (evidence B-R2).
Another immune activation pathway in asthma is IL-13. Molecules are already under development with this target in mind: tralokinumab and lebrikizumab with anti-IL-13 effect, and dupilumab61 (evidence B-R2) which is directed against the alpha subunit of the IL-4 receptor, a target shared by both IL-4 and IL-13. Lebrikizumab showed a greater positive effect in patients with elevated pre-treatment levels of circulating periostine62 (evidence B-R2). Trials have been conducted with pitrakinra, an IL-4 mutein, that works as an antagonist by inhibiting IL-4 and IL-13 binding to the shared IL-4Rα/IL-13Rα1 complex63 (evidence B-R2). Daclizumab, a humanized IgG1 monoclonal antibody against the IL-2R-α chain of activated T-lymphocytes, has been shown to be useful in moderate and severe asthma inadequately controlled on IC64 (evidence B-R2).
Molecules that act on the IL-17 (brodalumab) and the IL-17A receptor (secukinumab) in neutrophilic asthma are currently under investigation, since IL-17 induces IL-8 expression, the main neutrophil-attracting factor, but results to date have been disappointing65 (evidence B-R2).
Phenotype-based TreatmentIf the inflammatory phenotype is identified, treatment response can be predicted. In this respect, there is sufficient data to confirm that eosinophilic inflammation has a high predictive value for response to IC in patients with this type of airway disease, irrespective of their diagnosis. There is evidence for adjusting treatment in SUCA patients on the basis of induced sputum testing (in experienced clinics), although this could not be shown for FeNO66 (evidence A-R1).
Triamcinolone could be an alternative in patients with refractory eosinophilia,28 and its effect may be enhanced with low-dose theophylline.54 Therapeutic options, such as omalizumab, are available, and mepolizumab, reslizumab, lebrikizumab or dupilumab could be used in this patient group in the near future.
Some selected patients with neutrophilic asthma may be candidates for macrolide treatment.44
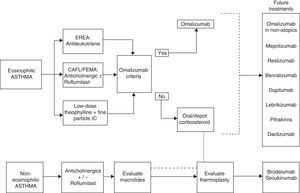
Tiotropium is another alternative for patients with CAFL.41 Finally, thermoplasty can be considered in carefully selected patients in experienced units (Fig. 3).
Severe Uncontrolled Asthma in ChildhoodLess than 5% of asthmatic children have SUCA. However, the care of children with SUCA accounts for more than twice the direct (medication, visit to the emergency room, hospitalization) and indirect resources (missing school, parents’ absenteeism from work, etc.) used by the others67 (evidence B-R1).
Since there are insufficient follow-up studies, no clear relationship can be established between asthma phenotypes in children and in adults, but severe asthma in childhood does seem to continue into adulthood, and very few individuals manage to control their disease.5
Most children referred to specialists for SUCA may belong to one of the following groups, or indeed more, as there may be some overlap, or a patient may be classified into different groups at different ages:
Persistent asthma, occurring most days, for at least 3 months, despite high-dose IC and other controllers, such as LABA, montelukast, and less frequently, low-dose oral theophylline or long-term SC.
Recurrent severe exacerbations requiring at least ICU admission or 2 hospital admissions, or more than 2 courses of SC in the last year, despite appropriate background treatment for asthma.
CAFL due to anatomical reduction of airway caliber, occurring either before birth (maternal smoking, hypertension or gestational diabetes) or postnatal (viral infections, reflux aspiration), detected by lack of improvement in respiratory function (FEV1<−1.96 of the Z score) after administration of 1 dose of IM triamcinolone or 2 weeks of oral SC (although the choice of SC and dose remain unclear)68 (evidence D-R2).
As in adults, if a child presents with apparent SUCA, a number of considerations must be made:
Is the Diagnosis of Asthma Correct?Other diagnostic possibilities must be considered, and extra tests performed, according to the degree of suspicion (Table 2).
Are the Prescribed Treatment and Compliance Adequate?SUCA was ruled out in 75% of children after a review of inhalation techniques and treatment compliance69 (evidence D-R2).
Are There Aggravating Environmental Factors?Both repeated exposure to airborne allergens to which the child is sensitized and exposure to tobacco smoke or atmospheric pollution contribute to poor asthma control, due to increased bronchial reactivity and reduced response to SC. If these factors are combined with a respiratory viral infection, severe asthma exacerbations may be easily triggered.
Are There Comorbidities?Rhinosinusitis, obesity, gastroesophageal reflux and psychosocial factors worsen asthma, although their degree of influence and mechanism of action are disputed. Food allergies behave more as a marker for severity, while breathing dysfunction syndromes may present concomitantly and lead to overtreatment if they are not correctly identified.
After analyzing the above-mentioned factors, an attempt should be made to determine if the patient has corticosteroid-sensitive SUCA (the most common pediatric presentation), or not (even if the patient has an eosinophilic inflammatory pattern in the airway), or if they have a neutrophilic or mixed pattern, or if there is CAFL.
To this end, the various tests listed in Table 6 should be scheduled in a series of visits. In the first visit, allergy tests should be repeated, to determine if the patient has become sensitized to other airborne allergens, and cotinine in saliva should be analyzed to detect exposure to tobacco smoke70 (evidence D-R2CPG).
Evaluation of Severe Uncontrolled Asthma in Children.
| Visit 1 | Visit 2(at 2 weeks) | Visit 3(at 2 weeks) | |
|---|---|---|---|
| ACT questionnaire | √ | √ | √ |
| Spirometry and bronchodilator test | √ | √ | √ |
| Induced sputum | √ | √ | √ |
| FeNO | √ | √ | √ |
| Bronchoscopy with BAL and endobronchial biopsy | √ | ||
| Triamcinolone IM | √ |
ACT: Asthma Control Test; FeNO: fractional exhaled nitric oxide; IM: intramuscular; BAL: bronchoalveolar lavage.
To confirm corticosteroid response, and since there is no agreement as regards the optimal dose, duration and route of administration in children, we suggest using triamcinolone, 40–80mg IM, depending on age and weight71 (evidence D-R2). Response should be confirmed 2 weeks later (Table 7). Responders are patients who improve in all 3 domains, non-responders are those who do not improve in any domains, and partial responders are those who improve in 1 or 2 domains. The latter category is the most common.37
Positive Response to Triamcinolone in Children.
| Clinical response: ACT questionnaire score | Score >20–25 points or increase ≥5 points |
|---|---|
| Lung function tests | FEV1 normal or increase >15%Negative bronchodilator test |
| Induced sputum | Normalization of eosinophil count |
| FeNO | <24ppb |
ACT: Asthma Control Test; FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1s.
Patients who respond to SC may benefit from omalizumab, which has been shown to be safe and effective in comparative trials in children over the age of 6.48 Any other treatment should also be maintained, including SC, until symptoms are controlled.
Non-responders who have persistent neutrophilic airway inflammation may benefit from macrolides, although there is insufficient evidence. Use of azithromycin has been suggested with the same regimen as for cystic fibrosis: 250mg/day for children weighing <40kg and 500mg/day for those >40kg, 3 times a week for 6 months, followed by a reevaluation of efficacy72 (evidence A-R1). Low-dose oral theophylline may also be tried: target blood concentrations are 5–10mg/l.
Conflict of InterestCarolina Cisneros Serrano has received fees for lectures and participation in advisory boards from Astra-Zeneca, GSK, Boheringer, Novartis, Takeda, ViforPharma and Chiesi. The other authors declare that they do not have any conflict of interests.
Please cite this article as: Cisneros Serrano C, Melero Moreno C, Almonacid Sánchez C, Perpiñá Tordera M, Picado Valles C, Martínez Moragón E, et al. Normativa sobre asma grave no controlada. Arch Bronconeumol. 2015;51:235–246.