Non-invasive mechanical ventilation (NIMV) is used to treat acute respiratory failure by improving gas exchange abnormalities and reducing the signs of respiratory effort, dyspnea and the activity of accessory respiratory muscles. Bronchoscopy is a key technique in the study of respiratory diseases that is necessary to perform in acute and critical patients, most of the times only after orotracheal intubation (OTI) due to possible complications of the technique. In this review, we evaluate the evidence of NIMV use during bronchoscopy, concluding that its use should be considered in severe patients as an alternative that is capable of preventing the complications related with OTI and mechanical ventilation, especially in patients with chronic obstructive pulmonary disease and with a tendency toward developing hypercapnia.
La ventilación mecánica no invasiva (VMNI) actúa en el tratamiento de la insuficiencia respiratoria aguda mejorando las alteraciones del intercambio gaseoso y reduciendo los signos de trabajo respiratorio, la disnea y la actividad de la musculatura respiratoria accesoria. La broncoscopia es una técnica clave en el estudio de las enfermedades respiratorias que es necesario practicar, en ocasiones en pacientes agudos o críticos; la única opción es ser realizada tras intubación orotraqueal (IOT), debido a las posibles complicaciones de la técnica. En la presente revisión evaluamos la evidencia del uso de la VMNI en el transcurso de la broncoscopia, concluyendo que su utilización debiera considerarse, en pacientes graves, como alternativa capaz de evitar complicaciones relacionadas con la IOT y la ventilación mecánica invasiva (VMI), especialmente en pacientes con enfermedad pulmonar obstructiva crónica y con tendencia a la hipercapnia.
Non-invasive mechanical ventilation (NIMV) applied in the treatment of acute respiratory failure improves gas exchange alterations and reduces the signs of respiratory effort, dyspnea and the activity of the accessory respiratory musculature while any drugs that are administered take effect.1 In this manner, patients may be more quickly stabilized and tracheal intubation (TI), invasive mechanical ventilation (IMV) and admittance to an intensive care unit (ICU) can all be avoided.2
Bronchoscopy is a fundamental technique used in the study of respiratory diseases. It provides visualization of the upper airway and initial divisions of the tracheobronchial tree and allows for samples to be taken from the trachea, bronchi, mediastinum and lung parenchyma.3 Furthermore, it is essential in the therapeutic management of patients with hemoptysis, aspiration of a foreign body, excess secretions, neoplastic lesions and obstruction of the central airway. Bronchoscopy has become a key element in modern pulmonology: it has diagnostic indications in most respiratory diseases and is the cornerstone in some aspects of current respiratory therapy methods.4 Among the different types of bronchoscopy, flexible bronchoscopy (introduced in 1968 by Ikeda et al.5) is the most widely used technique, although there are other types, such as rigid bronchoscopy (RB)6 or ultrasound-guided bronchoscopy (EBUS).7,8
Non-Invasive Mechanical Ventilation and BronchoscopyWhen considering bronchoscopy in patients under NIMV, acute cases (critical patients with the need for emergency or preferential interventions) should be differentiated from those with long-term or home NIMV who would require non-emergency or scheduled bronchoscopy.9
The first group includes patients who require preferential treatment, such as secretion suctioning, bronchoscopy-guided intubation10—especially in patients with swallowing alterations and risk for lung aspiration (myopathies with bulbar involvement)—or the extraction of foreign bodies.
The presence of abundant secretions in patients with acute respiratory failure is one of the causes for NIMV failure, which can contraindicate its use.11 However, bronchoscopy can facilitate the elimination of secretions, improve ventilation and avoid tracheal intubation. This therapeutic bronchoscopy has even been adapted to the NIMV in patients with pathologies such as cystic fibrosis with severe exacerbations and abundant secretions.12 It may also be used in patients with pulmonary infiltrates of unknown origin who may require microbiological samples to be taken, or those of another nature (diffuse alveolar hemorrhage or organizing pneumona13). Other indications for bronchoscopy in patients with NIMV include the study of atelectasis, hemoptysis, torpidly evolving pneumonia in immunosuppressed patients, suspicion of lung neoplasia, bronchopleural fistulas or tracheoesophageal fistulas.
Performing bronchoscopy presents potential complications that can be related with the procedure itself, individual patient factors or with the ability, qualifications and experience of the bronchoscopist.
The gas exchange alterations that occur during bronchoscopy can be triggered by the object of the bronchoscopic study, but the bronchoscope itself also causes dysfunctions as it takes up around 10% of the tracheal lumen—diminishing its caliber—, increases the resistance of the airway and reduces tidal volume. The impact of bronchoscopy on increased flow resistance will depend on the caliber of the respiratory tract and/or the size of the bronchoscope. The increased respiratory effort associated with increased resistive loading can precipitate acute respiratory failure in critical patients. In addition, the application of suction through the bronchoscope channel lowers airway pressure at the end of expiration, facilitating early alveolar closure. This may cause arterial oxygen pressure (PaO2) to decrease between 10 and 20mmHg during bronchoscopy.14 These changes persist after the procedure is completed, and the time that the gas exchange takes to normalize ranges from 15min in normal subjects and several hours in patients with lung disease. All this justifies the use of supplemental O2 in patients at risk for desaturation during bronchoscopy.15
Moreover, the bronchoscope itself has an irritating effect on the mucosa as it passes through the respiratory tract, which frequently generates cough, nausea, vomiting and laryngeal, tracheal or bronchial spasms. These reflexes, largely dependent on the cranial nerve pairs IX, X and XI, produce discomfort and neurovegetative overstimulation. This is counteracted with the administration of topical anesthesia, such as lidocaine, or with intravenous anesthetics, such as propofol, used in cases of allergy to the former.16,17 The administration of these topical anesthesia, saline solution or techniques like bronchoalveolar lavage (BAL) can worsen hypoxemia as a consequence of an altered ventilation–perfusion ratio.18 This can become aggravated when greater sedation is required with anesthesia due to the intolerance to the procedure.
In patients with chronic obstructive pulmonary disease (COPD) and other obstructive disease, bronchoscopy can promote air trapping as functional residual capacity increases up to 17% and auto-PEEP increases. These effects can be deleterious in patients with hypercapnic respiratory failure or in those who initiate with decompensation from their baseline disease.19
It should be mentioned that bronchoscopy is occasionally indicated in critical or high-risk patients in order to reach an etiologic diagnosis, either opting for TI to perform bronchoscopy or assuming empirical treatment in cases of infectious lung disease, aimed at avoiding the exploration. Taking into account that associations like the American Thoracic Society consider bronchoscopy a contraindication for BAL when a PaO2 of 75mmHg or an FiO2 of 90% are not reached during spontaneous ventilation with O2 supply, TI is usually opted for when bronchoscopy is essential.20 However, IMV entails a series of risks related with the insertion of the endotracheal tube, associated respiratory infections, intrinsic IMV problems or those observed after the withdrawal of the endotracheal tube. NIMV is an alternative that is able to avoid complications related with intubation and mechanical ventilation, especially in patients with COPD and a tendency toward hypercapnia.21 Spontaneous ventilation maintained during the procedure guarantees the balance of the V/Q ratio and hemodynamic stability, allowing for bronchoscopy to be done.22
It is well known that NIMV can maintain airway permeability, ease drainage of secretions and improve respiratory effort. It has been demonstrated that the simple administration of continuous positive airway pressure (CPAP) can improve minute volume and reduce the probability of atelectasis. This fact is more notable in patients with tracheomalacia.23 CPAP, which acts as a sort of “pneumatic stent” mechanism, can increase transmural pressure of the central airways, increasing its cross-sectional diameter. Nevertheless, this is not the only mechanism that can explain the improvement in ventilatory variables. Elevating lung volume increases peak expiratory flow, residual functional capacity and the effectiveness of cough, which is especially important in patients with neuromuscular disease or weakened respiratory musculature.24
Although CPAP is not strictly considered a NIMV system due to absence of inspiratory support, lower risk has been observed for acute respiratory failure after bronchoscopy in cases where this system has been used.25,26 Other similar studies showed improvement in the PaO2/FiO2 ratio at the expense of elevated PaO2 compared with patients treated with conventional oxygen therapy.27 The beneficial effect of NIMV in bronchoscopy has also been confirmed in patients with COPD effected by pneumonia and hypercapnic encephalopathy.28,29 In addition, it has been demonstrated that in moderate–severe hypercapnic encephalopathy of exacerbated COPD patients, short-term survival is similar when using NIMV or IMV, although there are fewer complications in the former.21
ProcedureSettingIt is usually recommended that the procedure be performed in an ICU or intermediate respiratory care setting, or a standard bronchoscopy room capable of dealing with any complications.19
Application of Non-Invasive Mechanical VentilationNIMV should be initiated in a previously non-ventilated patient at least 15 or 20min before bronchoscopy is begun.14,27
There are no published papers comparing the different NIMV methods. Initially, double-pressure systems were used with support mode (IPAP 17cm and EPAP 5cm) and FiO2 at 100%,30 but procedures have been described with lower inspiratory pressures and FiO2 at 70%.31 A support pressure of 10cm during the procedure is recommended.28 It has also been proposed to carry out bronchoscopy in a specific CPAP mode, specifically Boussignac CPAP, coupled to a face mask.26 This system is based on the so-called law of conservation of energy in motion, by which gas enters at high speed coming from a flow meter and passes through micro-capillaries. This generates acceleration in the form of microjets that cause the air molecules of the jet to transfer part of the kinetic energy to the air molecules situated in the CPAP body, which are thus accelerated (virtual valve or turbulence effect). The gas velocity transforms into pressure depending on the flow of gas provided by the flow meter.32
It is recommended to begin with IPAP at 14–15cm and EPAP at 5cm with bi-pressure systems (or 10cm of support pressure) and 5cm when CPAP is used, except if different parameters were previously established due to the characteristics of the patient.27,28 FiO2 is usually used at a level that would provide O2 saturation above 90%. On a practical level, this implies an FiO2 greater than 0.5 or a flow between 6 and 12l/min. Preferentially, FiO2 is usually started at 1 and then reduced according to patient tolerance.33 Obviously, in order to reach the desired pressure with the Boussignac CPAP model, the required level marked by the flow meter is used.
Generally, other parameters would include the S/T mode (spontaneous/timed), a rate of 4–8 mandatory inspirations and an I/E ratio (inspiration/expiration) according to the characteristics of the patient (usually 1:2, 1:3 in patients with airflow obstruction and 1:1 in restrictive patients). With respirators that allow for it to be monitored, expiratory tidal volume (Vt) should be at between 8 and 10ml/kg and respiratory rate always below 25min−1. Monitoring the pressure curve and flow can help assess patient–respirator interaction, although data are still needed to demonstrate its usefulness in the NIMV/bronchoscopy scenario.
CPAP MasksRegarding CPAP interfaces, almost all types of masks have been used. The most widely used are face masks,30,31 which provide for oral or nasal entry of the bronchoscope.
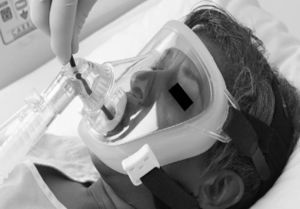
Currently, endoscopic face masks usually have two orifices: one for the administration of gas and a second orifice that is sealed and distensible and allows for an endoscope to be introduced (Figs. 1 and 2).
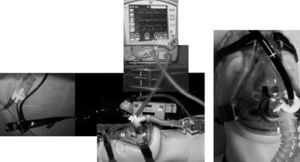
Some authors have proposed modifications in the interfaces based on the presence of a second orifice with a silicone diaphragm for the entry of the bronchoscope.34 There are also universal T connections for coupling with any type of mask (Fig. 3). When Boussignac CPAP is used for bronchoscopy, face masks are used.26 Antonelli described the possibility of performing bronchoscopy with NIMV using a helmet-type interface, which completely covers the patient's head to maintain ventilatory support35 (Fig. 4).
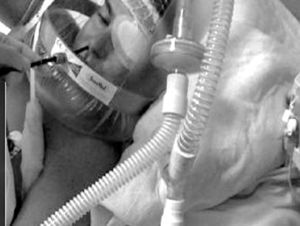
Heunks et al.36 has designed a system based on a complete face mask with the insertion of a synthetic plastic cylinder in the mask in order to avoid air leaks during bronchoscopy. Twelve hypoxemic patients (PaO2/FiO2: 192±23) underwent bronchoscopy with BAL without any important complications (Fig. 5). A similar system is shown in Fig. 6.
Variation of Fig. 5, with an oronasal mask.
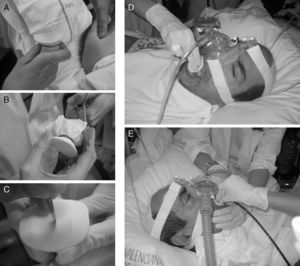
Chiner et al.37 have recently published the results of a prospective study with 35 patients that used a hand-made system of a membrane made out of a latex glove coupled with a bite block with a small incision. This maintains the pressure administered by BIPAP through a nasal mask, and bronchoscopy is carried out orally with good diagnostic and therapeutic results (Fig. 7).
Non-invasive mechanical ventilation carried out with a nasal mask and insertion of the bronchoscope through the mouth. (A) Bite block placed inside a latex glove and held with a suture. (B) Excess material is cut off. (C) Minimal incision in the membrane of the mouthpiece. (D) Introduction of the bite block under non-invasive mechanical ventilation. (E) Oral insertion of bronchoscope and technique carried out with non-invasive ventilation.
NIMV does not necessarily imply greater sedation. In fact, it is not unadvised, although it is essential to have the necessary experience in drug management. For topical anesthesia, lidocaine is usually used, following standard bronchoscopy procedure. Some authors propose the use of propofol for bronchoscopies done under NIMV, which reduces patient discomfort without significant adverse effects.38
Patient PositionIt is recommended to carry out the procedure with the patient in a semi-recumbent position, which is implied by the anterior entry of the bronchoscope. However, NIMV itself has been used in supine decubitus in order to optimize respiratory parameters in other explorations like transesophageal ultrasound or interventional cardiology (or in cases with diagnosis of dynamic airway collapse, which required maintaining the patient in this position).39–41
Nasal vs Oral InsertionThere are authors who have used both nasal and oral approaches. In many instances, this is determined by the mask used. With the case of face masks, nasal or oral pathways can be used.27,28 Oral insertion can also be used with modifications and systems coupled to a bite block, with the concomitant use of a nasal mask.42 In explorations with the helmet, either nasal or oral entry could be used, with the patient sitting or in supine decubitus.35 Likewise, the use of Boussignac CPAP, since it is an open system, allows for oral or nasal entry.26 The unintentional loss of pressure (interface) during bronchoscopy should be controlled in order to be able to guarantee NIMV effectiveness.
TechniquesThe techniques used are conditioned by patient characteristics. Bronchial suction, BAL or protected catheter are usually used for microbiological or cytological diagnosis.28,37 Although it is not formally contraindicated, some authors recommend not performing transbronchial biopsy while the patient is under mechanical ventilation due to greater risk for pneumothorax and bleeding.14,43–45
DurationAs in all interventions in high-risk patients, it is recommended to take the shortest time possible in order to achieve the objective. It usually varies, but the mean duration of bronchoscopy with NIMV in patients with pneumonia is approximately 8min.28
Post-Bronchoscopy CareNIMV will be maintained with parameters similar to those prior to bronchoscopy within 15 and 90min after the end of the procedure.12,23,29,41
ComplicationsSince NIMV offers inspiratory flow at positive pressures, patients may present with gastric distension and increased risk for bronchoaspiration. The abdominal pressure generated by intragastric distension can reduce functional residual capacity and add a restrictive component.46 NIMV requires a high degree of patient collaboration, which facilitates coupling to the respirator, reducing the probability of gastric distension and restrictive-type alveolar collapse.
There are also other complications related with bronchoscopy (desaturation, bleeding, poor collaboration, agitation, etc.), whose resolution is approached as in a standard bronchoscopy procedure.
Less frequently, there may be major cardiovascular complications (malignant arrhythmias, acute coronary syndrome, cardiac arrest), which can be minimized through proper patient selection and monitoring during bronchoscopy.
Immediate NIMV adjustments (hypoxemia, hypercapnia, maladjustment, discomfort) during the test are done according to standard practice, summarized below:
If there is hypoxemia, EPAP is increased 2×2cmH2O until an O2 saturation ≥90% is reached. It should be kept in mind that very high values (greater than 10) can cause greater risk for gastric distension and intolerance in the patient. If it persists, the flow or FiO2 is increased.
Hypercapnia is corrected by raising IPAP to the point that good pH margins are reached (maximum 25cmH2O) and by adjusting EPAP to avoid rebreathing.
Patient maladjustment is observed with certain signs or data: contraction of the sternocleidomastoid (which will condition IPAP), abdominal contraction or active expiration (which would recommend reducing IPAP), failed inspiration (which would justify the increase in EPAP) and low Vt (requiring the mask to be adjusted, avoiding a peak pressure higher than 30cmH2O and permitting leaks if the expired volume were adequate).47
Nasal or facial injuries secondary to the use of the mask are not included in this section as they appear after prolonged NIMV use (a minimum of several hours).
Persistent patient desaturation or clinical deterioration would require the procedure to be suspended and additional measures to be taken, including TI.
Therefore, it is important to emphasize that the procedure should be evaluated in an adequate setting by experts in bronchoscopy with fast access to TI if cardiopulmonary resuscitation were needed.48
ContraindicationsContraindications of this procedure are those of NIMV itself, such as cardiac arrest, severe encephalopathy, severe gastrointestinal bleeding, severe hemodynamic instability, a history of facial surgery or trauma, inability to guarantee a permeable airway or high risk for aspiration.22 Some related contraindications are psychomotor agitation, respiratory failure due to neurological causes or asthma state.33 Contraindications due to facial deformities or facial, esophageal or gastric interventions36 should also not be overlooked. Others, such as airway obstruction or the inability to cough up secretions, can become indications for bronchoscopy with NIMV.28 There are other absolute contraindications of bronchoscopy itself, such as the lack of collaboration, unstable angina, recent acute myocardial infarction (within the previous 20 days), severe arrhythmias, thrombopenia less than 60000 or prothrombin activity below 60% if biopsy is planned.49 Bronchial asthma is a relative contraindication with an FEV1 less than 60%.
The inability to maintain oxygen saturation above 85% despite maintaining high FiO2 is indicative of TI if bronchoscopy is being considered.50 Patient cooperation is essential, but if this is not possible, TI or empirical treatment should be chosen.33
Special SituationsOrotracheal Intubation With Bronchoscopy and Non-Invasive Mechanical VentilationIntubation with bronchoscopy is usually done as an emergency procedure in the ICU or as a scheduled procedure in the anesthesia department in cases of difficult intubation.
This can be dangerous due to the risk for secondary hypoxia after the apnea induced by general anesthesia. NIMV has been used for bronchoscopy in hypoxemic or hypercapnic patients and its use could be considered for tracheal intubation with bronchoscopy, including predictably difficult intubations, as long as the technique is strictly followed.51
The masks used are face masks with 2 orifices, as previously commented. These masks provide an FiO2 of 1 and ensure the gaseous mix with no leaks. In addition, with ventilation with positive pressure, distension of the pharyngeal–tracheal structures is obtained during inspiration and visualization of the glottis is improved during bronchoscopy. By adding positive end-expiratory pressure (PEEP), the glottis is maintained open during expiration.52
Many intubations are nasotracheal, and in these cases the technique entails applying local anesthesia to the nostrils and oropharynx, placement of the endoscopic mask over the face, connection to the respirator and, finally, entry of the bronchoscope through the previously lubricated orotracheal tube until reaching 3cm from the carina. At this time, some authors administer midazolam if the saturation with NIMV surpasses 94%.53 Other authors prefer administering midazolam once the tip of the endotracheal tube is visualized when it surpasses the distal end of the bronchoscope passes. During the progression of the endotracheal tube using the bronchoscope as a guide, the scope will be set so it does not come out of the trachea due to the inertia of the endotracheal tube progressing along the bronchoscope. If resistance is felt while sliding the endotracheal tube, it could be useful to slightly withdraw the tube and then reinsert it while slightly rotating clockwise.54 Once the tube is inside the trachea, the balloon is inflated, the bronchoscope is withdrawn, the mask is disconnected and the endotracheal tube is connected to the respirator.
TI with bronchoscopy through an endoscopic face mask, but with manual ventilation, is a technique described by certain groups. It is a technique that is used when intubation with laryngoscope has not been possible, but with no history of previous use of NIMV.55
Occasionally, bronchoscopy can be done after TI or nasotracheal intubation with a thin endotracheal tube, introducing the bronchoscope parallel to and outside of the endotracheal tube without inflating the sleeve. This provides for simultaneous ventilation of the patient, as long as the leak amount is tolerable. It can be used for quick procedures that involve transitory IMV (maintaining the endotracheal tube) or NIMV (reverting sedation and extubation) after completion. As we have commented, these procedures should be done by experienced teams and in the ICU or similar settings.19,48
Pediatric PatientsPediatric patients are at a high risk for hypoxemia and hypercapnia during bronchoscopy due to the smaller diameter and the greater tendency for collapse of their airways. In addition, there is increased resistance in the airways created by the bronchoscope itself.56 Any obstruction due to edema, lesion occupying space or elements that reduce the cross-sectional diameter for the bronchoscope will exponentially increase the resistance to gas flow (ley de Hagen–Poiseuille), limiting ventilation by significant reductions in tidal volume, peak inspiratory pressure and expiratory flow.57
CPAP increases the width of the laryngeal space and reduces the tendency toward collapse of the lateral walls of the pharynx, which are the most sensitive structures of the upper airway.58 Bronchoscopy in pediatric patients with spontaneous breathing has been associated with a decrease in tidal volume and respiratory flow, which can be reverted with the use of CPAP. In pediatric patients with tracheomalacia, where pulmonary volumes are low and resistance to expiratory flow is high, NIMV improves lung volumes and expiratory flow.59
Sleep Apnea Hypopnea Syndrome/Obesity Hypoventilation Syndrome/Morbid ObesityIn these patients, the hypoxemia triggered during bronchoscopy, increased on occasion by the effect of sedation, can be truly important, to the point that, even without it, hypercapnia while awake may worsen.60 NIMV counteracts negative inspiratory pressures and the hypotonicity of the upper airway muscles of these patients, applying positive pressure on the oropharynx.33 In addition, in this type of patients it has been described that, under conditions of moderate sedation, the collapse of some areas like the hypopharynx can limit the visualization of the structures through the bronchoscope.61 The positive pressure generated by NIMV allows for the laryngeal structures to be identified as the device passes the hypopharynx and is introduced through the vocal chords. This aspect is fundamental in these cases, which are usually considered as having difficult intubation.62
Rigid Bronchoscopy and Non-Invasive Mechanical VentilationMajor applications for rigid bronchoscopy include therapeutic bronchoscopy (laser, electrocautery or cryotherapy), the placement of tracheobronchial stents, tumor resection, dilation of tracheobronchial stenosis and the extraction of foreign bodies, particularly in children. Other indications are the treatment of hemoptysis and taking deep biopsies, obtained with the rigid biopsy forceps and completed with other techniques like bronchial cryotherapy.6,63
The application of intermittent positive pressure and jet ventilation are the 2 standard ventilatory modes that guarantee effective ventilation during RB.64 Patients can also be controlled with assisted spontaneous breathing, but this may cause low levels of respiratory acidosis.
Some authors have proposed the use of negative-pressure ventilation (NPV), a NIMV mode, as a system of optimal ventilation for RB procedures. With the aim to assess the effectiveness of NPV using a poncho wrap system during the application of laser therapy in endobronchial lesions, Vitacca developed a randomized, controlled, prospective study that confirmed its usefulness in patients with apnea. During the laser therapy procedure under general anesthesia, this system avoided the development of hypercapnia, secondary respiratory acidosis and the increased need for O2 supply.65 In a later paper, the use of NPV in patients undergoing rigid bronchoscopy demonstrated a reduced use of opioids, shorter recovery time, less respiratory acidosis and less need for assisted manual ventilation and O2.66
Endobronchial UltrasoundEndobronchial ultrasound (EBUS) is a minimally invasive diagnostic technique that complements flexible bronchoscopy. It is a cutting-edge tool that combines traditional video-assisted endoscopy and ultrasound, which can obtain ultrasound images of the structures outside the bronchial walls, such as lymph nodes.67 There are 2 different EBUS techniques, depending on the type of transducer: radial EBUS and linear EBUS.
The technique is done under sedation by the bronchoscopist or anesthetist. After placing an oropharyngeal cannula in the mouth, the ultrasound bronchoscope is inserted. To date, there are no prospective studies published about the advantage of using NIMV in ultrasound bronchoscopy, although its future use is likely, probably with the application of high-frequency NIMV modalities.
Conclusions and Practical Recomendations27,28,48,68,69NIMV is a safe therapeutic procedure that allows for complementary tests like bronchoscopy to be carried out, with ventilatory support that provides efficacy similar to IMV. However, it must be supervised by expert staff, and on occasion it requires an ICU or intermediate respiratory care unit setting with proper monitoring, NIMV management and preparation if TI or IMV were necessary.
Once it has been decided to do bronchoscopy with NIMV, NIMV treatment should be initiated previously and its efficacy in the patient verified. Patient cooperation is essential (except in children or scheduled intubations).
Bronchoscopy should be done by an experienced bronchoscopist, guaranteeing a precise, quick test. In cases where improvement in the gas exchange is not observed after previous treatment with NIMV or inability to maintain oxygen saturation above 85% despite maintaining high FiO2, TI should be scheduled without delay and should be done under the best possible conditions.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Esquinas A, et al. Broncoscopia durante la ventilación mecánica no invasiva: revisión de técnicas y procedimientos. Arch Bronconeumol. 2013;49:105–12.