Primary ciliary dyskinesia (PCD) is a congenital disease characterized by impaired ciliary function, which involves a wide range of symptoms that are mainly respiratory. Recent articles that base diagnosis on ciliary ultrastructural studies have estimated its prevalence to be 1/10000 newborns, which is higher than previously thought. Our objective is to define criteria for clinical suspicion of DCP that would indicate ultrastructural studies, while optimizing these and providing early diagnoses.
MethodsOurs is a descriptive, retrospective review of patients diagnosed with DCP in the Pediatric Pulmonology Unit at our hospital, from 1994 to 2010. The inclusion of cases was based on clinical suspicion criteria. Diagnosis was confirmed by characteristic ultrastructural changes observed in the electron microscopic study of the cilia.
ResultsSixty-three samples were analyzed, and 34 cases of DCP and 1 case of acilia were confirmed. Mean age at diagnosis was 3.6 (range: 1 month to 19 years of age). The most frequent initial symptom was prolonged neonatal tachypnea in the newborns (20%) and lower respiratory tract episodes in the school-aged patients: recurrent pneumonia (46%), difficult-to-control asthma (26%), bronchiectasis (8.6%) and massive atelectasis (2.9%). Associated symptoms were present in 46% of the cases.
ConclusionOur publication proposes the implementation of several clinical criteria depending on the age of presentation that would indicate the active search for alterations in the ciliary epithelium at reference centers.
La discinesia ciliar primaria (DCP) es una enfermedad caracterizada por disfunción de las células ciliadas que se manifiesta con una sintomatología muy variable, principalmente respiratoria. Trabajos recientes que basan el diagnóstico en el estudio ultraestructural ciliar calculan una prevalencia mayor de la estimada previamente, situándola en 1/10.000 nacidos vivos. Nuestro objetivo es definir unos criterios clínicos de sospecha de DCP que sirvan de indicación para dicho estudio, lo que permitiría optimizarlo y realizar un diagnóstico precoz.
MétodosRevisión retrospectiva de los pacientes diagnosticados de DCP en la Unidad de Neumología Infantil del hospital entre 1994 y 2010. La inclusión de los casos se determinó a partir de criterios clínicos de sospecha. El diagnóstico se confirmó con la observación de cambios ultraestructurales característicos en el estudio ciliar por microscopia electrónica.
ResultadosSe analizaron 63 muestras y se confirmaron 34 casos de DCP y un caso de acilia. La edad media del diagnóstico fue de 3,6 años (rango de un mes a 19 años). La clínica inicial más frecuente fue taquipnea neonatal prolongada en los recién nacidos (20%) y cuadros de vías respiratorias bajas en los pacientes en edad escolar: neumonías recurrentes (46%), asma de difícil control (26%), bronquiectasias (8,6%) y atelectasia masiva (2,9%). En el 46% de los casos existían síntomas asociados.
ConclusionesSe propone la aplicación de un determinado número de criterios clínicos dependiendo de la edad de presentación que indiquen la búsqueda activa de una alteración en el epitelio ciliar en centros de referencia.
Primary ciliary dyskinesia (PCD) is a mainly autosomal recessive hereditary disease, characterized by a dysfunction of the hair cells present in the respiratory and gonad tissue, among other locations. The incidence of the condition is reckoned, on the basis of radiographic studies and clinical observations in the stage prior to electron microscope examination, to be 1/15000–20000 live births. According to these data, the approximate incidence of the Kartagener syndrome would be in the region of 1/30000 to 1/40000, and 50% of the cases of PCD would present situs inversus. However, recent studies in which diagnosis is based on ultrastructural ciliary study, show a larger number than has been accepted hitherto, estimating it at 1/10000 live births.1,2
PCD is one of a group of conditions in which the respiratory hairs are mobile (ciliary motility syndrome), ciliary movement is dyskinetic and ineffectual (PCD), or the cilia are absent (ciliary aplasia), the latter of which is extremely rare.1,3,4 In 1976, Afzelius5 described, as the origin of the condition, the absence of dynein arms in the microtubules of the of the bronchial cilia and of the sperm flagella.
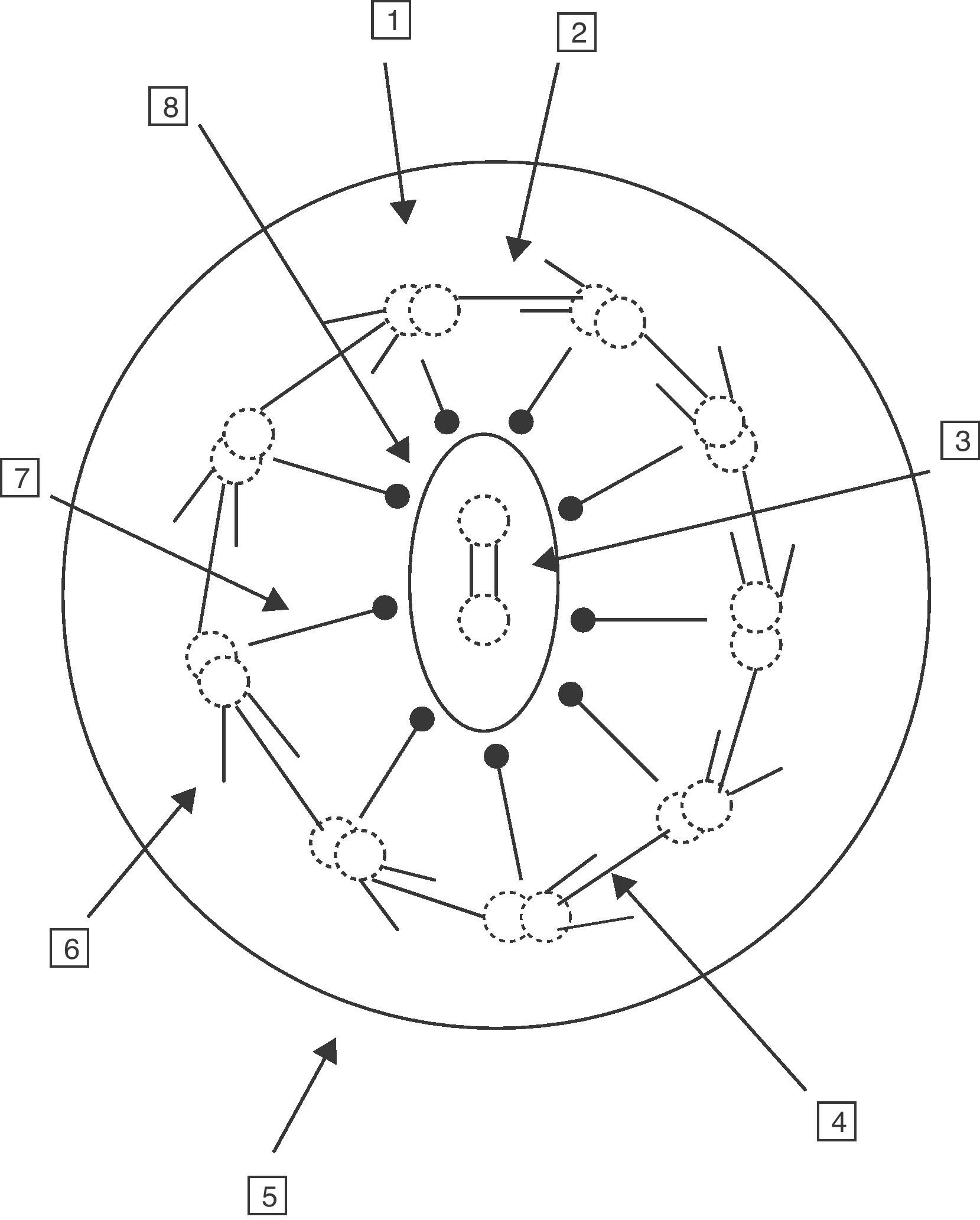
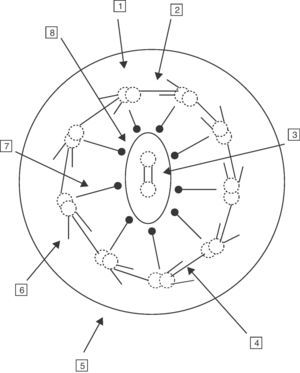
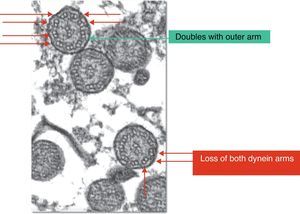
A normal hair cell is made up of 250 proteins organized around an axoneme or set of microtubules which extend from the cytoplasm to the far end of the cilium. The normal cilium consists of a central pair of microtubules surrounded by a sheath and 9 other external doublets forming a characteristic “9+2” pattern. The dynein complexes are associated with the peripheral doublets, the nexine bridges hold the peripheral doublets together and the radial spokes connect the central pair with the peripheral microtubules. The microtubules and their associated proteins are anchored in the apical cytoplasm of the cell by a complex of 9 microtubule triplets. The role of the respiratory cilia is to perform a coordinated beat with an appropriate frequency and pattern for the clearing of secretions and the elimination of residue from the airways (Fig. 1).6–8
There are mobile hair cells in the respiratory epiletium (as there also are in the paranasal sinuses and the middle ear), the ependyma of the brain, the vas deferens and ovarian tubes, and the epididymis, among other locations, as a result of which this pathology is characterized by a great phenotypic variability. The dysfunction of the ciliary motility complicates mucociliary clearance (one of the respiratory system's defense mechanisms), which accounts for the fact that these patients are more susceptible to chronic respiratory infection from birth. This condition also affects the sperm flagellum and the cilia of the fallopian tubes, frequently resulting in sterility in men and reduced fertility in women. The inefficiency of the nodal cilia temporarily present during the development of the embryo leaves the asymmetry of the internal organs at the mercy of chance, for which reason 50% of these patients suffer from total situs inversus.9
PCD is a heterogeneous condition in which numerous genes and proteins are involved and which, for this reason, manifests itself in a range of symptoms. Currently at least 10 genes related to this pathology are known to us.10–13 However, these mutations can only be studied in specially equipped laboratories, and most of the genes involved are yet to be identified, so we still seem to be far from the possibility of molecular diagnosis.
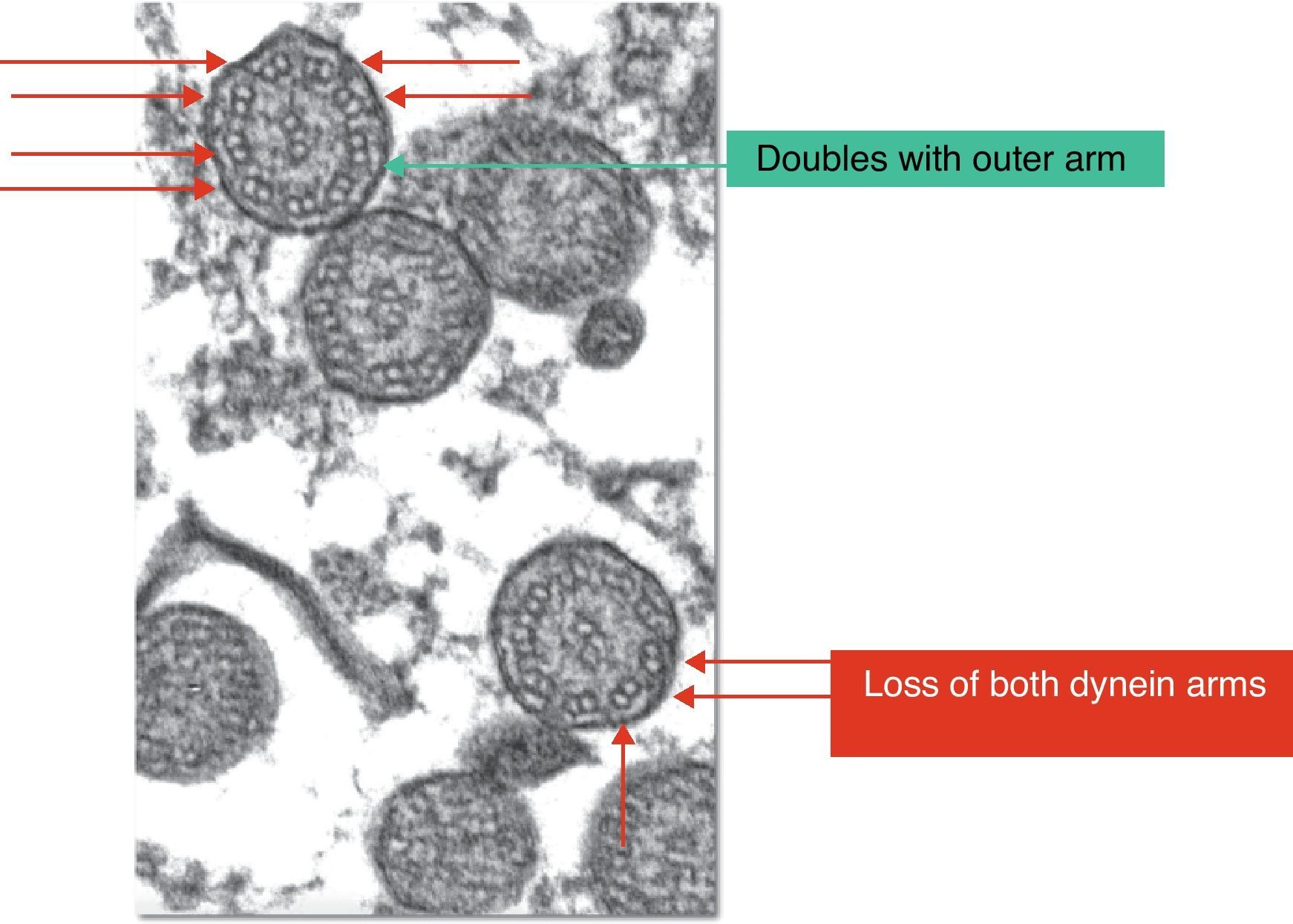
PCD diagnosis is currently based on functional and structural study of the cilia obtained from samples of nasal mucus (Fig. 2).14,15
Our aim is to come up with a set of clinical criteria on suspicion of PCD that might serve as an indication for further ultrastructural study, which in turn would help to optimize such study and thus result in a diagnosis as quickly as possible. This would make it possible to initiate treatment at an early stage and to implement a program of clinical monitoring which, while they will not be able to prevent the development of the disease, would prove effective in controlling it since, as it is borne out by previous publications, pulmonary function at the time of diagnosis is significantly worse when the said diagnosis is carried out when the patient is an adult.4
Among the available therapeutic measures the most important are monitoring of the general state of the patient and the respiratory and auditory functions, physiotherapy and physical exercise aimed specifically at aiding the drainage of secretions and an appropriate treatment of respiratory infection, with the application, is the event of such infection, of the antibiotic indicated by the sputum culture and bronchoalveolar lavage.16
Materials and MethodsA retrospective descriptive study is based on a review of the clinical histories of patients suspected of having PCD in the Children's Pulmonology Unit of the Hospital del Mar between 1994 and 2010. The aim is to determine clinical criteria applicable in the event of suspicion of PCD which might serve as an indication for the performance of complementary tests. The diagnosis was confirmed in the observation of characteristic ultrastructural changes in ciliary examination by electron microscope.
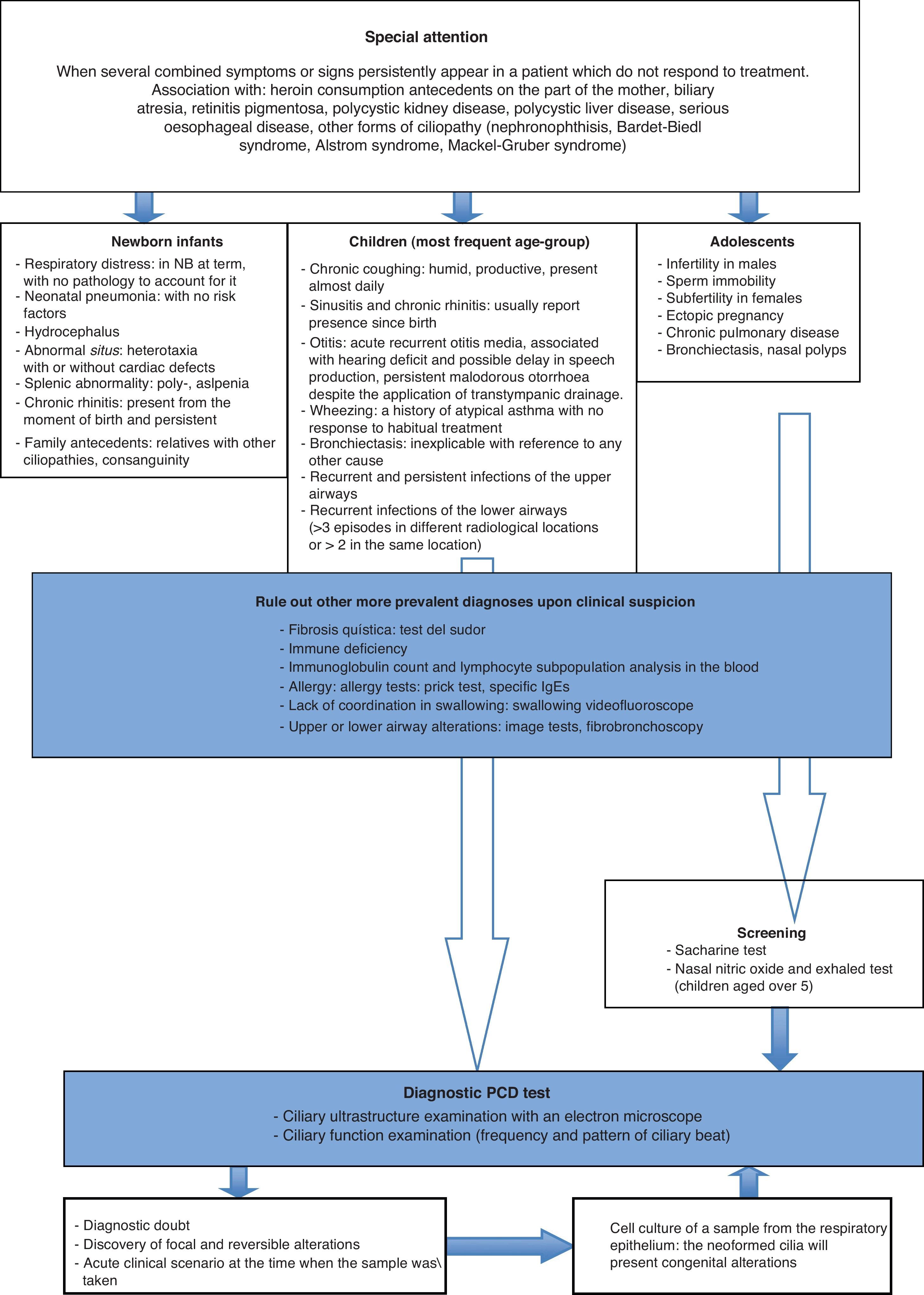
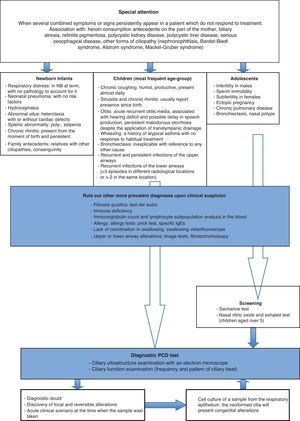
The cases used included in the study were selected on the basis of clinical criteria for the suspicion of PCD (Fig. 3), and the decision was made to not only exclude patients with a previous diagnosis for other pathologies such as active rhinitis, acute respiratory infection and those to whom topic nasal treatment had been administered in the previous 4 weeks, but also patients from whom invalid samples had been obtained.13
If patients suspected of having PCD, the following tests were carried out: thorax radiography, blood-tests to determine the presence of alfa-1-antitripsina, the sweat test, allergy tests, immunoglobulin count, and in some cases a high resolution CT scan, with a view to ruling out other more frequent pathologies, depending on the clinical profile in each case. A respiratory epithelium sample was obtained by means of brushing in the region of the inferior nasal meatus or the nasal septum, following washing of the nostrils with physiological saline, in order to study the ciliary ultrastructure with an electron microscope. For the brushing in each nostril an endocervical cytobrush was used, which was then placed in a 2% glutaraldehyde solution for conservation and transferred to the laboratory of the Pathological Anatomy Service in a maximum time lapse of 2h. The samples were prepared for ultramicroscopic analysis by means of a 1% osmium tetroxide fixation, a buffer solution lavage and resin inclusion. Cuts of 150nm, dyed with lead citrate and uranyl acetate, were used. They were examined in an electron microscope (Phillips CM 100) with angular goniometer to adjust the angulation of the sample, with samples being considered valid if more than 100 cilia could be observed. Samples in which more than 50% of the cilia presented characteristic anomalies according to the criteria of Afzelius (Fig. 2) and of the European Respiratory Society were diagnosed as alterations of the ultrastructure that were consistent with the presence of PCD.6,13 Structural alterations that are regarded as a sign of PCD are the loss of one or both dynein arms. Other anomalies of the ciliary structure that have been described and are considered to cause ciliopathies include: absence or defect of the radial arms, the absence or dislocation of the central microtubules and anomalies in the position of the peripheral microtubules, supranumerary doublets, the absence of the axoneme and disorganization in the orientation of the cilia.3,9 If less than 50% is affected, the anomalies observed could be a physiological finding (anomalies observed in less than 10% of the cilia examined) or they could be secondary to, for example, virus infections, an inflammation of the airway or exposure to toxic substances. Despite the fact that some of the findings observed in secondary ciliary dyskinesia are similar to alterations characteristic of primary ciliary dyskinesia, the fact that the said modifications are focal and reversible suggests the secondary type.17
Results63 samples were examined, with 34 cases of PCD being confirmed and 1 case of acilia (this high percentage of positive results, far superior to other series, can be accounted for by the high level of suspicion concerning the condition in our center and the fact that the patients were carefully selected). The remaining 28 samples produced negative results. The average age at diagnosis was 3.6 years (range, 1 month to 19 years). Among the patients diagnosed there were 23 males (65.7%) and 12 females (34.3%). The newly born baby boy with dextocardia, also presented bilateral pyelic ecstasia, balanic hypospadias, and partial trigonocephaly due to premature closure of the metopic suture.
Eight cases were newly born infants (22.86%), 7 of whose mothers had consumed heroin during gestation18 accidental and 1 was diagnosed as a result of the fortuitous discovery of dextocardia (with situs solitus). The most frequent initial clinical evaluation was prolonged neonatal tachypnea in the newly born infants, 7 cases (20%).
In the school-age patients the most frequent problems were in the lower respiratory tracts, with the following being among the most prevalent: recurring pneumonia, 15 cases (46%); barely controllable asthma, 9 cases (26%); bronchiectasis, 3 cases (8.6%); and massive atelectasis, 1 case (2.9%). 46% of the patients presented associated symptoms, the.
The application of alfa-1-antitripsina and the use of a sweat test were determined for all the children.
Microscopic analysis of the respiratory epithelium samples obtained from the nasal mucous showed, in all patients, ultrastructural alterations of the cilia which were consistent with a PCD diagnosis: the loss of both dynein arms, both internal and external, in 20 cases (57%), loss of the internal dynein arm in 13 cases (37.1%), the loss of the external dynein arm in 1 case (2.9%) and acilia in 1 case (2.9%). The average percentage of loss was 74% (range 50%–100%). Furthermore, in 10 of the patients other ultrastructural ciliary alterations were observed: disorganization of doublet distribution in 7 cases, loss of 1 tubulus in 2 cases and the loss of the tubulus in 2 cases and of the central doublet in 1 case.
DiscussionIn the absence of findings that are very characteristic of PCD such as situs inversus, the presentation is very unspecific, and could indicate the presence of repeated upper airway infection, which are typical scenario in pediatric cases, and would not normally arouse any suspicion of a possible PCD. Nevertheless, in these cases a number of very distinctive clinical particularities became apparent. The symptoms tend to be present from birth, with a tendency to become chronic and fail to respond to habitual treatments. What is more, in view of the extensive distribution of ciliary cells throughout the body, the simultaneous appearance of several different symptoms is in the same patient is typical of the condition.9 The situs invertus, while it is indeed a characteristic symptom, is not necessarily associated with all the PCD phenotypes. In most patients, the ciliary alterations are located in the respiratory tract, which is the reason why most of the symptoms are located in this region. Finally, there have been cases of the Kartagener syndrome in which the ciliary ultrastructure is normal.19
Chronic respiratory infection is the most habitual manifestation of the condition, but the possibility of other more common diagnoses should be considered before embarking on a ciliary study. Cistic fibrosis must be ruled out by means of the sweat test, immunodeficiency by means of the immunoglobulin count and the lymphocyte subpopulations in the blood, respiratory allergy by means of the prick test and specific IgE testing, and gastroesophageal reflux secondary pulmonary aspiration with pHmetry, among others (Fig. 3).
As screening assays, the saccharine test and/or the exhaled and nasal nitric oxide test can be used. The saccharine test assesses the mucociliary clearance, and when positive it indicates the need for a ciliary dyskinesia study. However, it is not useful in the case of small children or uncooperative patients as it requires that the patients be seated for 1h and a subjective judgment is given, as it is based on the recognition of the taste of saccharine.4,20,21 The exhaled and nasal nitric oxide test consists in detecting the diminution of this substance which occurs with PCD. Nasal air is more discriminatory than exhaled air, although it is difficult to carry this test out with small children.7,22 Diminution can also occur in cystic fibrosis, bronchiectasis, chronic sinusitis, and the Young syndrome.4 In view of this low specificity, in order to find a low value for nasal nitric oxide it is necessary to carry out other complementary examinations.23,24 High values belie a PCD diagnosis, but they do not allow it to be ruled out if other clinical data point to the presence of this disease. Nevertheless, the test is included in the diagnostic algorithm in some cases (with children aged over 5 years old and adolescents).
For confirmation of a PCD diagnosis, studies of ciliary function and ultrastructure are carried out. These tests must be conducted after a positive result has been obtained in the assay for PCD in adults. However, the difficulty involved in the execution of such tests in pediatric patients has resulted in their being recommended after a clinical suspicion of the disease has been established, and when other more frequent conditions with similar manifestations have been ruled out, as was the case in our series of patients. The examination carried out for the purpose of confirmation in our case was the ultrastructural analysis of the cilia with an electron microscope, a technique permitting a high degree of specificity, and available in leading centers.
The most common findings in this such an examination are the absence of dynein arms, defects in the radial bridges and anomalies in the position of the microtubules, observations which coincide with those found in our series. Afzelius25 propose different subgroups within PCD, depending on the characteristics of the ciliary dysfunction. The typical subgroup is characterized by the absence of dynein arms and/or an anomalous layout of the microtubules, which were also the most prevalent findings in our study. Another subgroup shows a cilium with an apparently normal structure but which fails to operate correctly. In other cases, the epithelium has microvilli cells but no cilia appear to be present (acilia syndrome or ciliary aplasia), an alteration which was diagnosed in one of our patients.
The examination of the ciliary ultrastructure as a method for the diagnosis of PCD is limited in that it can reveal anomalies which are in fact due to a ciliary alteration which is the result of damage to the epithelium caused by exposure to temperature changes, humidity or infections. For this reason, in the selection of the patients in our study, the prior diagnosis of another pathology, the active presence of rhinitis, acute respiratory infection or the administration of topic nasal treatment during the previous 4 weeks were all seen as criteria for exclusion from the study. Another way of preempting this limitation is to obtain a culture of the epithelium sample, as the cilia of the new generation will only reproduce the congenital and not the acquired defects.26
The examination of the ciliary function is based on the analysis of the frequency and the ciliary beat pattern by means of images obtained with a high resolution and high speed digital video camera attached to the microscope, and the subsequent analysis of these images with a computer program. As it was the case with the ultrastructural study, it is possible to obtain neoformed hair cells following the sample culture, which will only present the congenital abnormalities.
Given a strong suspicion of PCD, the analysis of the cilia by means of an electron microscope is one of the essential diagnostic techniques. This analysis is to be recommended even in those cases where the functional test shows normal ciliary frequency and beat pattern. However, between 10% and 28% of patients with PCD present a normal ultrastructure.9 This suggests that the functional study and the ultrastructural study should complement each other in order to arrive at a definitive diagnosis.
One detail in our series that is worth mentioning is that in the 7 cases corresponding to newly born infants of mothers who had consumed heroin during gestation, the clinical manifestation which prompted the structural analysis of the cilia was persistent tachypnea which was not attributable to a drug abstinence syndrome nor to any other cause. The incidence of ciliar alterations among newly born infants exposed to heroin prenatally was higher than among those not so exposed. This suggests that, over and above a certain degree of genetic susceptibility, prenatal exposure to heroin could have deleterious effects, in that it affects the normal development of the cilia and gives rise to alterations that are consistent with PCD.18
ConclusionsCurrently the PCD diagnostic tests are carried out more frequently, both due to the greater knowledge that we have of this condition and because of the availability of the means to conduct such tests in the leading centers. However, the variability of its phenotypic expression makes it difficult to establish sufficient suspicion of the disease for the analytical process to be initiated. Our study proposes the application of a specific number of clinical criteria, depending on the age at which certain symptoms make their appearance which, after other causes, such as immunodeficiency or cystic fibrosis, might indicate the need for an active search for an alteration in the ciliary epithelium in a leading center (Fig. 3).13,27,28 This will make it possible to better select patients for an ultrastructural study with a greater probability that the result will be positive, and in this way to improve the diagnosis of this underdiagnosed pathology.29 The early detection of these patients will facilitate the provision of a series of therapeutic resources which will maintain an adequate pulmonary activity for as long as possible, with the consequent decline in morbidity and increase in the patients’ quality of life.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Busquets RM, et al. Discinesia ciliar primaria: criterios clínicos de indicación de estudio ultraestructural. Arch Bronconeumol. 2013;49:99–104.