The precise cause of antibody-mediated immune responses on chronic obstructive pulmonary disease (COPD), asthma, and lung function remains unclear. We characterized the relationship between antibody-mediated immune responses to COPD, asthma, and lung function, ultimately achieve the prevention or treatment.
MethodsWe obtained summary data from published genome-wide association studies, including antibody-mediated immune responses, COPD, asthma, forced expiratory volume in the first second (FEV1), forced expiratory volume (FVC), and FEV1/FVC. Bidirectional two-sample mendelian randomization (MR) analysis was used to assess causal relationships of antibody-mediated immune responses, COPD, asthma, FEV1, FVC, and FEV1/FVC.
ResultsA total of 20 antibody-mediated immune responses were identified have a significant causal effect on COPD, asthma, FEV1, and FVC, with six exhibiting reverse causality. Importantly, the results of the five MR analyses were almost identical with respect to the causal effect of anti-polyomavirus 2 IgG seropositivity and varicella zoster virus glycoprotein E and I antibody levels on the risk of COPD, asthma, FEV1, and FVC.
ConclusionsThis study contributes to existing knowledge by investigating the causal relationship between antibody-mediated immune responses and respiratory conditions, including COPD, asthma, and lung function, using a two-sample MR design. The key findings can aid in identifying individuals at risk of these conditions and facilitate early prevention and diagnosis.
Chronic obstructive pulmonary disease (COPD) and asthma are two common progressive lung diseases, asthma affects more than 300 million people worldwide and its prevalence is increasing,1 and COPD is the third leading cause of death worldwide.2,3 Asthma and COPD are complex inflammatory diseases of the airways, which possibly related to impaired host defenses lead to increased susceptibility to pathogens, pollutants and allergens.4 Decreased lung function is the main clinical feature of asthma and COPD. Immune system may be a key driver in the pathogenesis of COPD, and the immune response is significantly associated with acute exacerbations of COPD.5 Evidence from observational studies suggests that more immune cells are present in the lung tissue of COPD and asthma sufferers compared to healthy individuals, along with a significant immune response.6 Mendelian randomization (MR) studies found that a variety of immune cells and inflammatory factors are risk factors for the development of COPD and asthma.7,8 However, changes in immune cells are the process by which an immune response occurs in the body, and immune cells recognise foreign substances such as antigens and activate, proliferate and differentiate to form effector cells and produce effector molecules to remove the foreign substances. In this process, foreign substances such as antigens are the initiators of the body's immune response, while pathogenic microorganisms are the most common cause of the body's immune response. Antibody-mediated immune responses persist in all stages of asthma and COPD from initial to end-stage,9 but whether there is a clear causal relationship between the development of COPD and asthma remains unclear. It is suggested that antibody-mediated immune responses may be causally related to the development of COPD and asthma. Guillaume et al. measured serological measurements of 20 different microorganisms in 9724 participant serum samples, selected the ones with a seroprevalence of >15% for genome-wide association studies (GWAS).10 Whether these detected antibody-mediated immune responses are causally associated with the development of COPD, asthma, and reduced lung function remains unclear. MR is a potential method of causal inference aimed at estimating the effect of exposure factors on outcomes and being able to control for potential confounders, thus avoiding reverse causation bias.11
The objective of this study was to investigate the causal relationship between antibody-mediated immune responses and COPD, asthma and lung function by bidirectional two-sample MR analysis.
MethodsStudy DesignThe study flow chart is shown in Fig. 1. First, we obtained summary data from published GWAS, including features such as antibody-mediated immune responses, COPD, asthma, forced expiratory volume in the first second (FEV1), forced expiratory volume (FVC), and FEV1/FVC ratio (Supplementary Table S1). Second, two-sample MR analysis was used to assess causal relationships of antibody-mediated immune responses to COPD, asthma, FEV1, FVC, and FEV1/FVC ratio. Finally, reversed two-sample MR analysis was used to explore the COPD, asthma, FEV1, FVC, and FEV1/FVC ratio on antibody-mediated immune responses. Our MR study was performed in accordance with STROBE-MR guidelines (Supplementary Table S2).12
Data SourcesThe GWAS summary statistics of antibody-mediated immune responses were retrieved from the large single homogenous population cohort, more than 500,000 British adults were recruited by CDC between 2006 and 2010, of whom 9724 provided samples of geranium for the measurement of 20 different micro groups.13
The GWAS summary statistics of COPD were retrieved from UK biobank with 2115 European ancestry cases, and 454,233 European ancestry controls.14 Considering that COPD subtypes emphysema and bronchiectasis may have different pathogenesis, we also analyzed the causal relationship between emphysema, bronchiectasis and exposure.14 Also, asthma has shown association with COPD and may have the same pathogenesis and pathological basis, so we also analyzed the causal relationship between asthma and exposure.14
The GWAS summary statistics of lung function, including FEV1, FVC, and FEV1/FVC, were retrieved from 475,645 European ancestry individuals, which is the largest global genomics study of lung function to date, with genome-wide association analyses of FEV1, FVC, and FEV1/FVC in 49 cohorts, adjusting for age, age squared, sex, and height, and applying genomic controls using the linkage disequilibrium (LD) score regression intercept.15
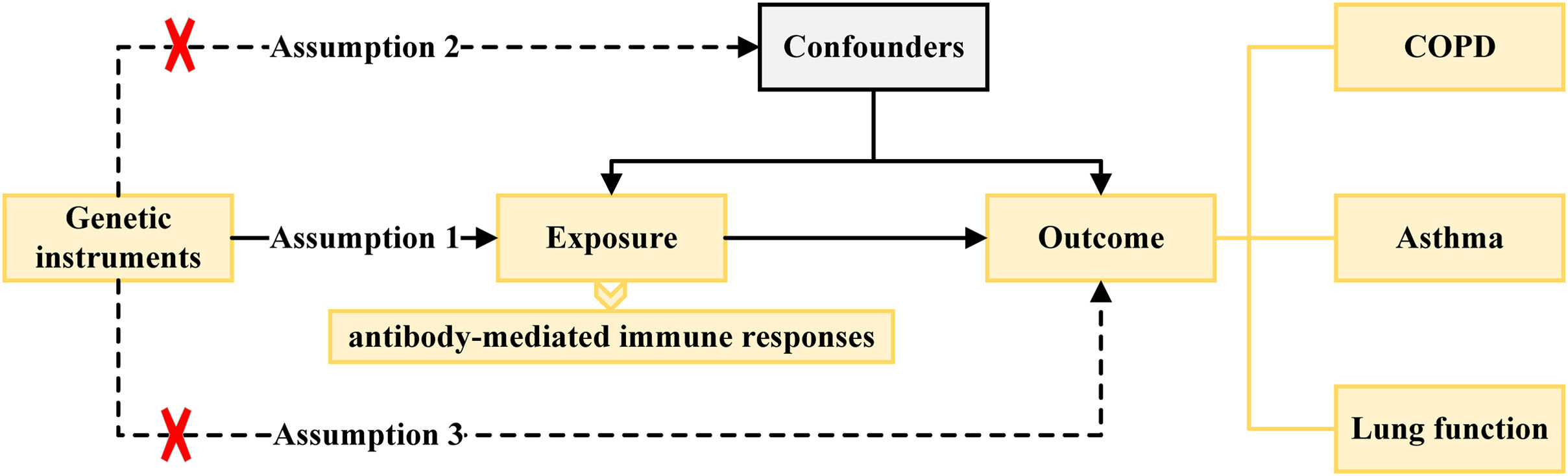
Instrumental Variable SelectionThe causal effects of MR analysis are based on three basic assumptions of genetic variation, including: (1) instrumental variables (IVs) are related to exposure factors; (2) IVs are not related to confounding factors; (3) IVs are not related to outcome variables and only affects the outcome variable through exposure factors.
The locus-wide significance threshold of SNP was P<1×10−5, and set P<1×10−5 if the number of single nucleotide polymorphism (SNP) screened after de-linked imbalance is less than 5 for antibody-mediated immune responses, and COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC ratio.16 The SNPs with impact of linkage disequilibrium were removed by clumping process (r2<0.001, window size=10,000kb). Even if each IV satisfies the three assumptions of the instrumental variables, chance may cause the estimates of the IVs to differ significantly from the expected results. Therefore, we used the MR pleiotropy residual sum and outlier (MR-PRESSO) test, and the outliers removed, respectively, to detect horizontal pleiotropy (MR-PRESSO outlier test P-values <0.05), and eliminated the pleiotropy effect (the SNPs were eliminated one by one according to horizontal pleiotropy until the MR-PRESSO global test P-value >0.05).17 SNPs related to outcome variables were excluded (P<0.05). The strength of the selected SNPs was evaluated using F-statistic, where SNPs with F-statistic <10 were excluded to avoid weak instrument bias in the MR analysis.18
Statistical AnalysisTwo-sample Mendelian RandomizationFive MR method was used to evaluate the causal relationship among the antibody-mediated immune responses, and COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC ratio. The inverse variance-weighted (IVW) was primary method to identify the causal relationship due to IVW usually provides the highest statistical power. The IVW method, inspired by meta-analytic methods, statistically combines or weighted linear regressions of the Wald ratio estimates across IVs, with less variance through weighted averaging in favor of the most statistically powerful results.19 And, in response to the possible heterogeneity and genetic pleiotropy of IV in MR analyses, the IVW approach can also assess the differences between causality estimates specific to each locus. In the absence of multinomially, IVW provides virtually unbiased estimates of causality.20 To obtain more conservative and robust estimates, we all used the random effects IVW test. The maximum likelihood,21 MR-Egger,22 weighted median,23,24 and weighted mode were as second methods to infer causal relationship.
The method of reverse MR was used to explore the reverse causality of COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC ratio on antibody-mediated immune responses, and the methods and steps of the analysis were the same as before. All MR analyses were performed in R (version 4.3.1) software, using the “TwoSampleMR” (version 0.5.7) (https://github.com/MRCIEU/TwoSampleMR) and “MR-PRESSO” (version 1.0) (https://github.com/rondolab/MR-PRESSO) packages.17
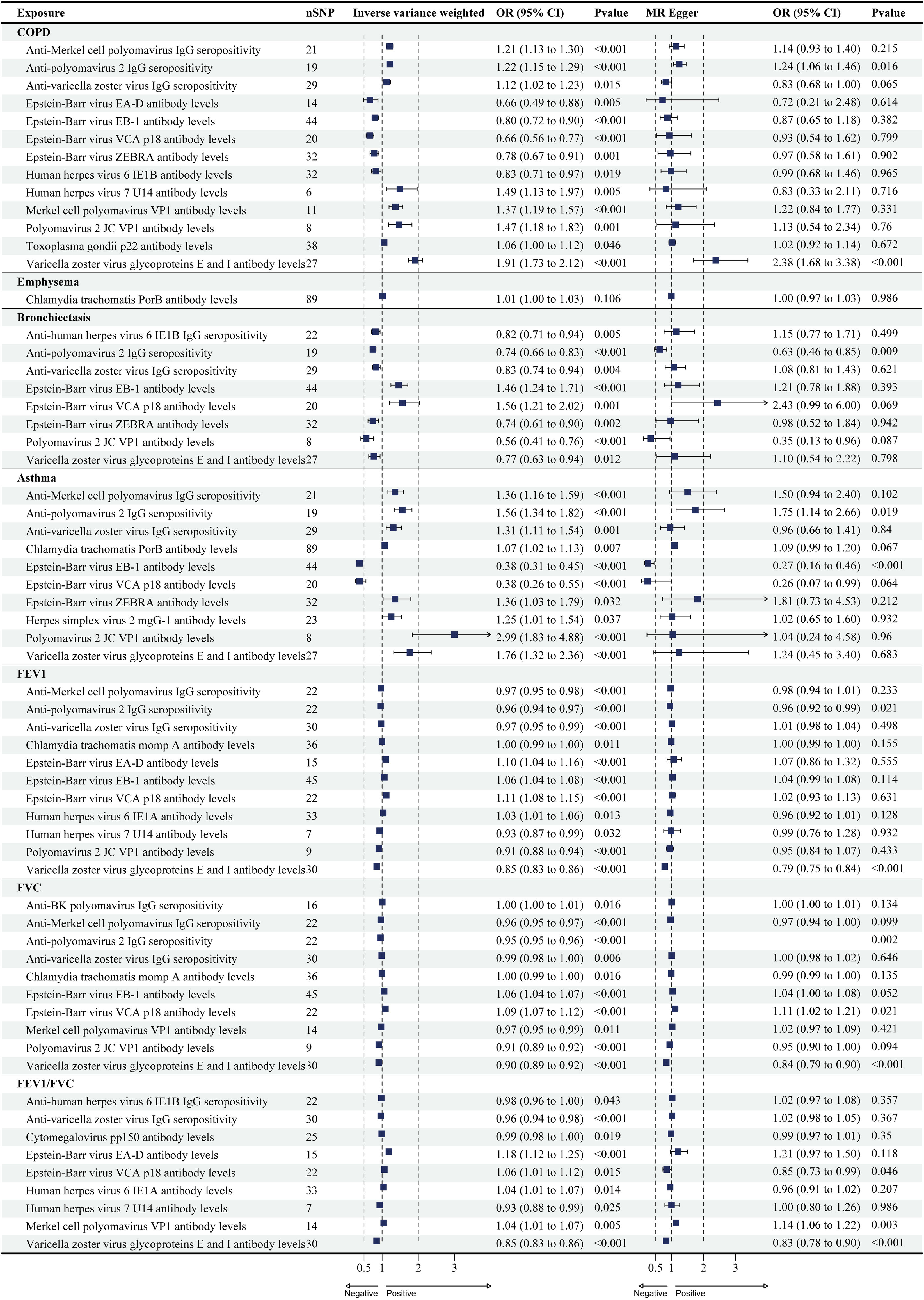
ResultsCausal Effects of Antibody-mediated Immune Responses on COPDA total of 13 antibody-mediated immune responses were identified as causally associated with COPD (PIVW<0.05, Fig. 2), and six did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05). Importantly, all five analyses showed that varicella zoster virus glycoproteins E and I antibody levels (odds ratio (OR)=1.91, 95% CI (1.73–2.12), P<0.001), and anti-polyomavirus 2 IgG seropositivity (OR=1.22, 95% CI (1.15–1.29), P<0.001) were a significant risk factor for COPD (Table 1 and Fig. 3; Supplementary Tables S3 and S4). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
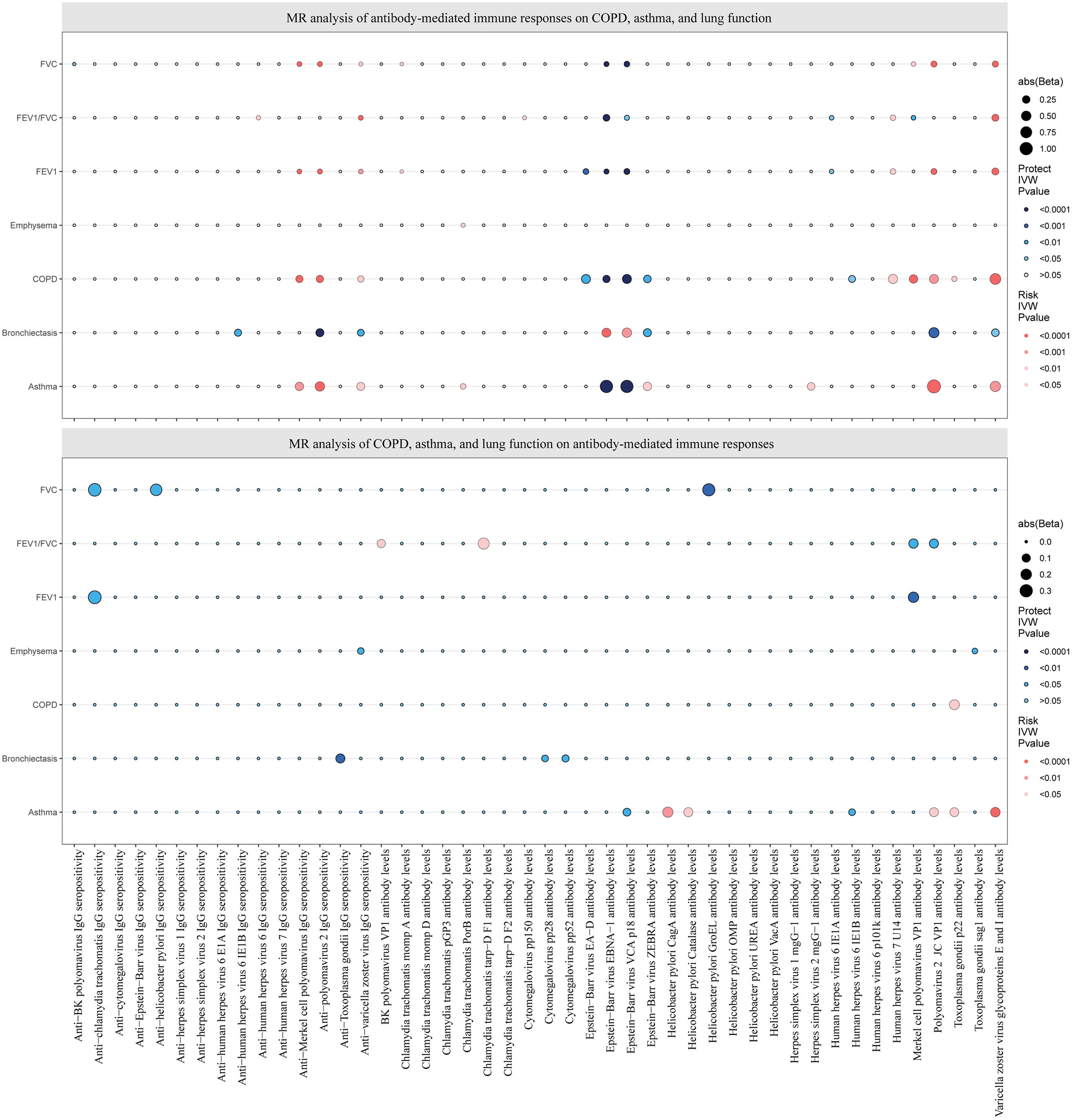
Causal estimates of bidirectional MR between antibody-mediated immune responses, COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC. Top: Estimates from the IVW analysis of antibody-mediated immune responses on COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC. Bottom: Estimates from the IVW analysis of COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC on antibody-mediated immune responses. The antibody-mediated immune responses features underlined in red were related to COPD, asthma, FEV1, and FVC. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MR: Mendelian randomization.
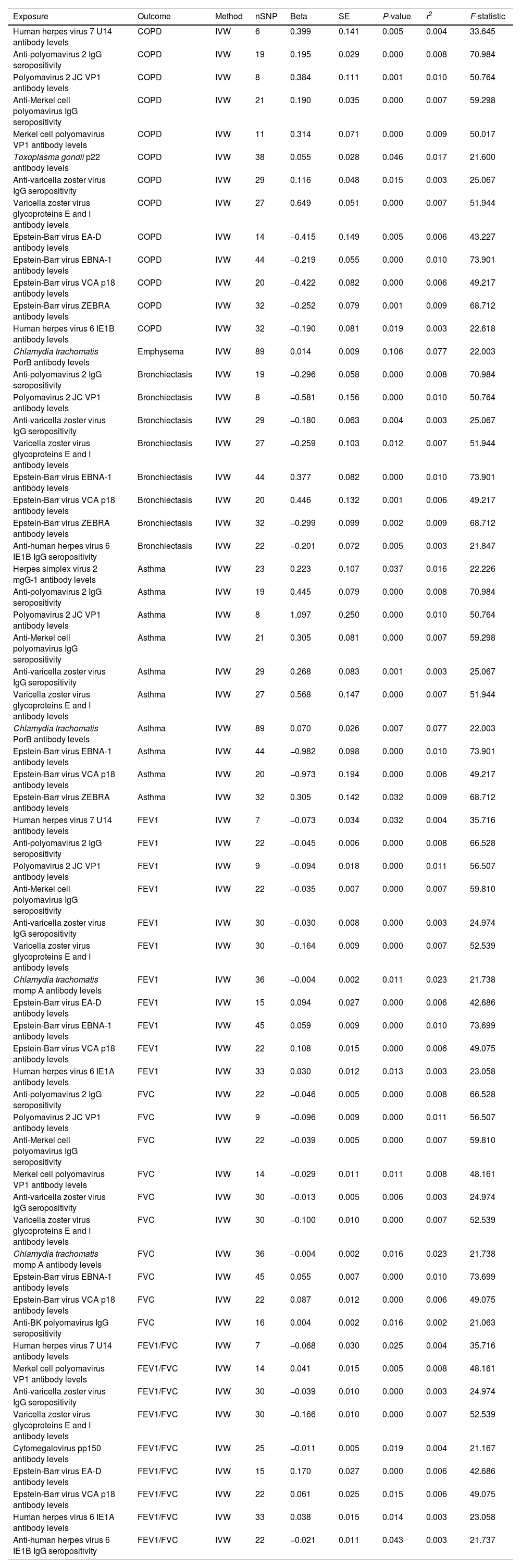
MR Analysis of Antibody-mediated Immune Responses on COPD, Asthma, and Lung Function.
| Exposure | Outcome | Method | nSNP | Beta | SE | P-value | r2 | F-statistic |
|---|---|---|---|---|---|---|---|---|
| Human herpes virus 7 U14 antibody levels | COPD | IVW | 6 | 0.399 | 0.141 | 0.005 | 0.004 | 33.645 |
| Anti-polyomavirus 2 IgG seropositivity | COPD | IVW | 19 | 0.195 | 0.029 | 0.000 | 0.008 | 70.984 |
| Polyomavirus 2 JC VP1 antibody levels | COPD | IVW | 8 | 0.384 | 0.111 | 0.001 | 0.010 | 50.764 |
| Anti-Merkel cell polyomavirus IgG seropositivity | COPD | IVW | 21 | 0.190 | 0.035 | 0.000 | 0.007 | 59.298 |
| Merkel cell polyomavirus VP1 antibody levels | COPD | IVW | 11 | 0.314 | 0.071 | 0.000 | 0.009 | 50.017 |
| Toxoplasma gondii p22 antibody levels | COPD | IVW | 38 | 0.055 | 0.028 | 0.046 | 0.017 | 21.600 |
| Anti-varicella zoster virus IgG seropositivity | COPD | IVW | 29 | 0.116 | 0.048 | 0.015 | 0.003 | 25.067 |
| Varicella zoster virus glycoproteins E and I antibody levels | COPD | IVW | 27 | 0.649 | 0.051 | 0.000 | 0.007 | 51.944 |
| Epstein-Barr virus EA-D antibody levels | COPD | IVW | 14 | −0.415 | 0.149 | 0.005 | 0.006 | 43.227 |
| Epstein-Barr virus EBNA-1 antibody levels | COPD | IVW | 44 | −0.219 | 0.055 | 0.000 | 0.010 | 73.901 |
| Epstein-Barr virus VCA p18 antibody levels | COPD | IVW | 20 | −0.422 | 0.082 | 0.000 | 0.006 | 49.217 |
| Epstein-Barr virus ZEBRA antibody levels | COPD | IVW | 32 | −0.252 | 0.079 | 0.001 | 0.009 | 68.712 |
| Human herpes virus 6 IE1B antibody levels | COPD | IVW | 32 | −0.190 | 0.081 | 0.019 | 0.003 | 22.618 |
| Chlamydia trachomatis PorB antibody levels | Emphysema | IVW | 89 | 0.014 | 0.009 | 0.106 | 0.077 | 22.003 |
| Anti-polyomavirus 2 IgG seropositivity | Bronchiectasis | IVW | 19 | −0.296 | 0.058 | 0.000 | 0.008 | 70.984 |
| Polyomavirus 2 JC VP1 antibody levels | Bronchiectasis | IVW | 8 | −0.581 | 0.156 | 0.000 | 0.010 | 50.764 |
| Anti-varicella zoster virus IgG seropositivity | Bronchiectasis | IVW | 29 | −0.180 | 0.063 | 0.004 | 0.003 | 25.067 |
| Varicella zoster virus glycoproteins E and I antibody levels | Bronchiectasis | IVW | 27 | −0.259 | 0.103 | 0.012 | 0.007 | 51.944 |
| Epstein-Barr virus EBNA-1 antibody levels | Bronchiectasis | IVW | 44 | 0.377 | 0.082 | 0.000 | 0.010 | 73.901 |
| Epstein-Barr virus VCA p18 antibody levels | Bronchiectasis | IVW | 20 | 0.446 | 0.132 | 0.001 | 0.006 | 49.217 |
| Epstein-Barr virus ZEBRA antibody levels | Bronchiectasis | IVW | 32 | −0.299 | 0.099 | 0.002 | 0.009 | 68.712 |
| Anti-human herpes virus 6 IE1B IgG seropositivity | Bronchiectasis | IVW | 22 | −0.201 | 0.072 | 0.005 | 0.003 | 21.847 |
| Herpes simplex virus 2 mgG-1 antibody levels | Asthma | IVW | 23 | 0.223 | 0.107 | 0.037 | 0.016 | 22.226 |
| Anti-polyomavirus 2 IgG seropositivity | Asthma | IVW | 19 | 0.445 | 0.079 | 0.000 | 0.008 | 70.984 |
| Polyomavirus 2 JC VP1 antibody levels | Asthma | IVW | 8 | 1.097 | 0.250 | 0.000 | 0.010 | 50.764 |
| Anti-Merkel cell polyomavirus IgG seropositivity | Asthma | IVW | 21 | 0.305 | 0.081 | 0.000 | 0.007 | 59.298 |
| Anti-varicella zoster virus IgG seropositivity | Asthma | IVW | 29 | 0.268 | 0.083 | 0.001 | 0.003 | 25.067 |
| Varicella zoster virus glycoproteins E and I antibody levels | Asthma | IVW | 27 | 0.568 | 0.147 | 0.000 | 0.007 | 51.944 |
| Chlamydia trachomatis PorB antibody levels | Asthma | IVW | 89 | 0.070 | 0.026 | 0.007 | 0.077 | 22.003 |
| Epstein-Barr virus EBNA-1 antibody levels | Asthma | IVW | 44 | −0.982 | 0.098 | 0.000 | 0.010 | 73.901 |
| Epstein-Barr virus VCA p18 antibody levels | Asthma | IVW | 20 | −0.973 | 0.194 | 0.000 | 0.006 | 49.217 |
| Epstein-Barr virus ZEBRA antibody levels | Asthma | IVW | 32 | 0.305 | 0.142 | 0.032 | 0.009 | 68.712 |
| Human herpes virus 7 U14 antibody levels | FEV1 | IVW | 7 | −0.073 | 0.034 | 0.032 | 0.004 | 35.716 |
| Anti-polyomavirus 2 IgG seropositivity | FEV1 | IVW | 22 | −0.045 | 0.006 | 0.000 | 0.008 | 66.528 |
| Polyomavirus 2 JC VP1 antibody levels | FEV1 | IVW | 9 | −0.094 | 0.018 | 0.000 | 0.011 | 56.507 |
| Anti-Merkel cell polyomavirus IgG seropositivity | FEV1 | IVW | 22 | −0.035 | 0.007 | 0.000 | 0.007 | 59.810 |
| Anti-varicella zoster virus IgG seropositivity | FEV1 | IVW | 30 | −0.030 | 0.008 | 0.000 | 0.003 | 24.974 |
| Varicella zoster virus glycoproteins E and I antibody levels | FEV1 | IVW | 30 | −0.164 | 0.009 | 0.000 | 0.007 | 52.539 |
| Chlamydia trachomatis momp A antibody levels | FEV1 | IVW | 36 | −0.004 | 0.002 | 0.011 | 0.023 | 21.738 |
| Epstein-Barr virus EA-D antibody levels | FEV1 | IVW | 15 | 0.094 | 0.027 | 0.000 | 0.006 | 42.686 |
| Epstein-Barr virus EBNA-1 antibody levels | FEV1 | IVW | 45 | 0.059 | 0.009 | 0.000 | 0.010 | 73.699 |
| Epstein-Barr virus VCA p18 antibody levels | FEV1 | IVW | 22 | 0.108 | 0.015 | 0.000 | 0.006 | 49.075 |
| Human herpes virus 6 IE1A antibody levels | FEV1 | IVW | 33 | 0.030 | 0.012 | 0.013 | 0.003 | 23.058 |
| Anti-polyomavirus 2 IgG seropositivity | FVC | IVW | 22 | −0.046 | 0.005 | 0.000 | 0.008 | 66.528 |
| Polyomavirus 2 JC VP1 antibody levels | FVC | IVW | 9 | −0.096 | 0.009 | 0.000 | 0.011 | 56.507 |
| Anti-Merkel cell polyomavirus IgG seropositivity | FVC | IVW | 22 | −0.039 | 0.005 | 0.000 | 0.007 | 59.810 |
| Merkel cell polyomavirus VP1 antibody levels | FVC | IVW | 14 | −0.029 | 0.011 | 0.011 | 0.008 | 48.161 |
| Anti-varicella zoster virus IgG seropositivity | FVC | IVW | 30 | −0.013 | 0.005 | 0.006 | 0.003 | 24.974 |
| Varicella zoster virus glycoproteins E and I antibody levels | FVC | IVW | 30 | −0.100 | 0.010 | 0.000 | 0.007 | 52.539 |
| Chlamydia trachomatis momp A antibody levels | FVC | IVW | 36 | −0.004 | 0.002 | 0.016 | 0.023 | 21.738 |
| Epstein-Barr virus EBNA-1 antibody levels | FVC | IVW | 45 | 0.055 | 0.007 | 0.000 | 0.010 | 73.699 |
| Epstein-Barr virus VCA p18 antibody levels | FVC | IVW | 22 | 0.087 | 0.012 | 0.000 | 0.006 | 49.075 |
| Anti-BK polyomavirus IgG seropositivity | FVC | IVW | 16 | 0.004 | 0.002 | 0.016 | 0.002 | 21.063 |
| Human herpes virus 7 U14 antibody levels | FEV1/FVC | IVW | 7 | −0.068 | 0.030 | 0.025 | 0.004 | 35.716 |
| Merkel cell polyomavirus VP1 antibody levels | FEV1/FVC | IVW | 14 | 0.041 | 0.015 | 0.005 | 0.008 | 48.161 |
| Anti-varicella zoster virus IgG seropositivity | FEV1/FVC | IVW | 30 | −0.039 | 0.010 | 0.000 | 0.003 | 24.974 |
| Varicella zoster virus glycoproteins E and I antibody levels | FEV1/FVC | IVW | 30 | −0.166 | 0.010 | 0.000 | 0.007 | 52.539 |
| Cytomegalovirus pp150 antibody levels | FEV1/FVC | IVW | 25 | −0.011 | 0.005 | 0.019 | 0.004 | 21.167 |
| Epstein-Barr virus EA-D antibody levels | FEV1/FVC | IVW | 15 | 0.170 | 0.027 | 0.000 | 0.006 | 42.686 |
| Epstein-Barr virus VCA p18 antibody levels | FEV1/FVC | IVW | 22 | 0.061 | 0.025 | 0.015 | 0.006 | 49.075 |
| Human herpes virus 6 IE1A antibody levels | FEV1/FVC | IVW | 33 | 0.038 | 0.015 | 0.014 | 0.003 | 23.058 |
| Anti-human herpes virus 6 IE1B IgG seropositivity | FEV1/FVC | IVW | 22 | −0.021 | 0.011 | 0.043 | 0.003 | 21.737 |
Note: COPD, asthma, FEV1, FVC. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced expiratory volume; MR: Mendelian randomization; IVW: inverse variance-weighted; SE: standard error.
Forest plots for causal effects of antibody-mediated immune responses on COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC. The horizontal bars correspond to the estimated OR with 95% CI using the IVW and MR-Egger method. COPD, asthma, FEV1, FVC. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MR: Mendelian randomization; OR: odds ratio; CI, confidence interval.
Only one antibody-mediated immune responses were identified as causally associated with emphysema (PIVW<0.05, Fig. 2), did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). The Chlamydia trachomatis PorB antibody levels were risk factor on emphysema, but there are differences in the results of different analyses (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
A total of eight antibody-mediated immune responses were identified as causally associated with bronchiectasis (PIVW<0.05, Fig. 2), and six did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). Importantly, all five analyses showed that anti-polyomavirus 2 IgG seropositivity (OR=0.74, 95% CI (0.66–0.83), P<0.001) was a significant protect factor for bronchiectasis (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
Causal Effects of Antibody-mediated Immune Responses on AsthmaA total of 10 antibody-mediated immune responses were identified as causally associated with asthma (PIVW<0.05, Fig. 2), and all trait did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). Importantly, all five analyses showed that anti-polyomavirus 2 IgG seropositivity (OR=0.74, 95% CI (0.66–0.83), P<0.001) was a significant risk factor for asthma, and Epstein-Barr virus EBNA-1 antibody levels (OR=0.38, 95% CI (0.31–0.45), P<0.001) was a significant protect factor for asthma (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
Causal Effects of Antibody-mediated Immune Responses on FEV1, FVC, and FEV1/FVC RatioA total of 11 antibody-mediated immune responses were identified as causally associated with FEV1 (PIVW<0.05, Fig. 2), and only one did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). All five analyses showed that anti-polyomavirus 2 IgG seropositivity (OR=0.96, 95% CI (0.94–0.97), P<0.001), and varicella zoster virus glycoproteins E and I antibody levels (OR=0.85, 95% CI (0.83–0.86), P<0.001) were a significant risk factor for FEV1 (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
A total of 10 antibody-mediated immune responses were identified as causally associated with FVC (PIVW<0.05, Fig. 2), and two did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). All five analyses showed that anti-polyomavirus 2 IgG seropositivity (OR=0.95, 95% CI (0.95–0.96), P<0.001), and varicella zoster virus glycoproteins E and I antibody levels (OR=0.90, 95% CI (0.89–0.92), P<0.001) were a significant risk factor for FVC, and Epstein-Barr virus VCA p18 antibody levels (OR=1.09, 95% CI (1.07–1.12), P<0.001) was a significant protect factor for FVC (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
A total of nine antibody-mediated immune responses were identified as causally associated with FEV1/FVC (PIVW<0.05, Fig. 2), and one did not show heterogeneity (PIVW>0.05, PMR-Egger>0.05), and horizontal pleiotropy (P>0.05) (Table 1; S3 and S4 in Supplementary). All five analyses showed that varicella zoster virus glycoproteins E and I antibody levels (OR=0.85, 95% CI (0.83–0.86), P<0.001) was a significant risk factor for FVC, and Merkel cell polyomavirus VP1 antibody levels (OR=1.04, 95% CI (1.01–1.07), P=0.005) was a significant protect factor for FVC (Fig. 3). The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S5.
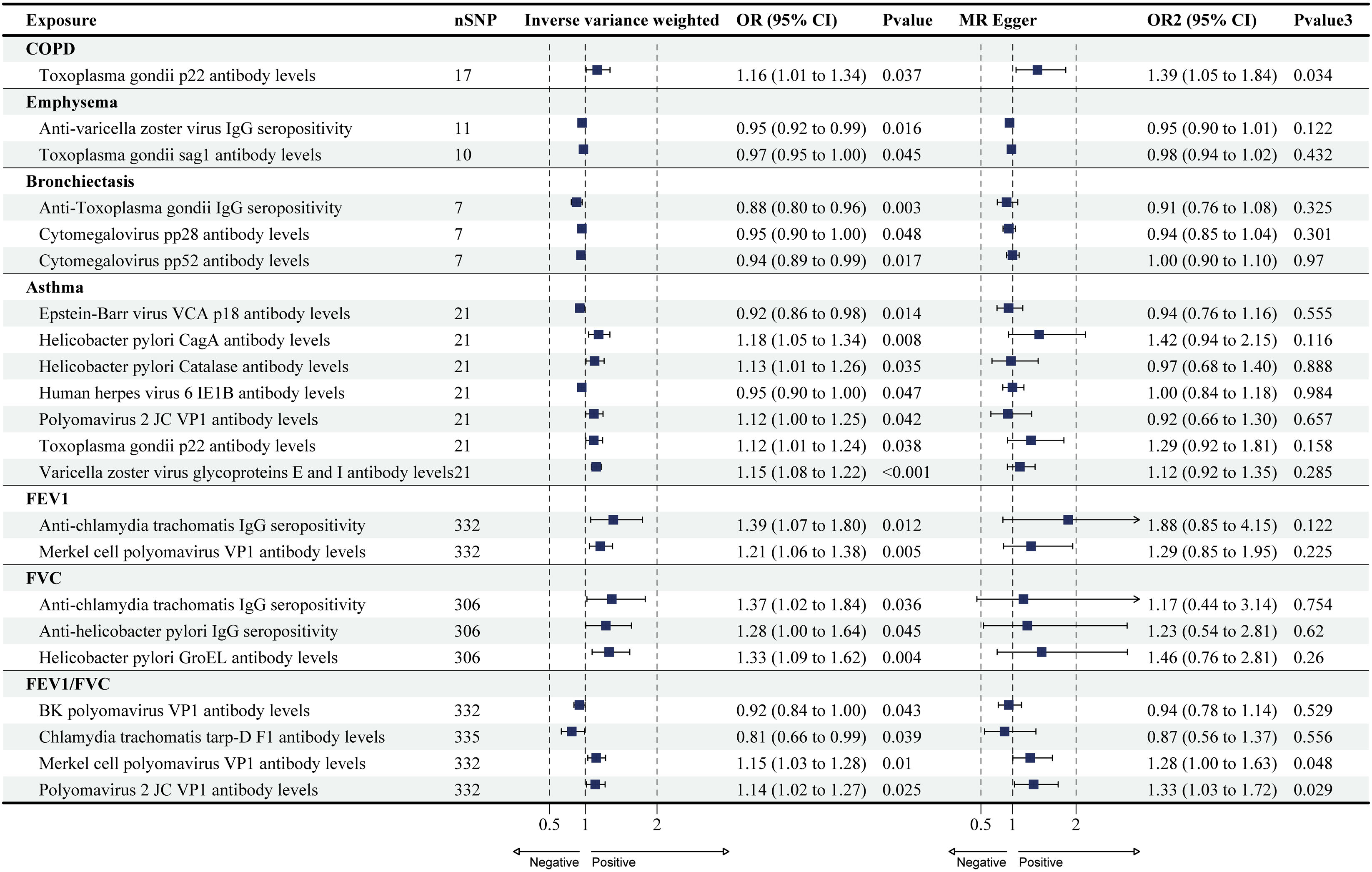
Causal Effects of COPD, Asthma, and Lung Function on Antibody-mediated Immune ResponsesThe reverse MR analysis found only a small number of causal relationships of COPD, asthma, and lung function on antibody-mediated immune responses, and these varied considerably when analyzed by different analytical methods (Figs. 2 and 4; Supplementary Tables S6 and S7), the results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S8. COPD, emphysema, bronchiectasis, asthma, FEV1, FVC, and FEV1/FVC were identified as causally associated with 1, 2, 3, 7, 2, 3, and 4 antibody-mediated immune responses (PIVW<0.05, Fig. 4), respectively. The results of Wald ratio analysis of individual SNPs are shown in Supplementary Table S8.
Forest plots for causal effects of COPD, asthma, emphysema, bronchiectasis, FEV1, FVC, and FEV1/FVC on antibody-mediated immune responses. The horizontal bars correspond to the estimated OR with 95% CI using the IVW and MR-Egger method. COPD, asthma, FEV1, FVC. COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MR: Mendelian randomization; OR: odds ratio; CI: confidence interval.
To our knowledge, this is the first study to analyses the causal relationship between antibody-mediated immune responses and COPD patients, asthmatics, and lung function. The results show that a total of 20 antibody-mediated immune responses that could have a significant causal effect on COPD, asthma, FEV1, and FVC. There was a total of seven antibody-mediated immune responses that could have a significant effect on COPD, asthma, FEV1, FVC at the same time, namely anti-Merkel cell polyomavirus IgG seropositivity, anti-polyomavirus 2 IgG seropositivity, anti-varicella zoster virus IgG seropositivity, Epstein-Barr virus EBNA-1 antibody levels, Epstein-Barr virus VCA p18 antibody levels, polyomavirus 2 JC VP1 antibody levels, and varicella zoster virus glycoproteins E and I antibody levels. More importantly, we find little difference between the results of the different analyses in terms of the risk causal impact of anti-polyomavirus 2 IgG seropositivity, and varicella zoster virus glycoproteins E and I antibody levels on COPD, asthma, FEV1, and FVC.
Infectious diseases are a major contributor to the global burden of disease and can have some impact on systemic diseases, such as fatigue, dyspnoea, pain, anxiety, depression and cognitive dysfunction following infection with COVID-19.25 And as this class of viruses is usually preventable, clarifying the causal relationship between viruses and COPD, asthma, and lung function will contribute to better clinical management and treatment of patients with COPD and asthma. The results of this study showed that polyomavirus and varicella zoster virus-induced immune responses were risk factors for COPD, asthma, FEV1, and FVC, and the results were consistent across the five MR analysis methods.
Polyomaviruses are small DNA viruses that are widely distributed in nature. Polyomaviruses usually infect immunocompromised individuals and can be transmitted through the respiratory system. Their infections are common and they do not show symptoms after infection.26 It has been shown that the CD8+ T cell response is specific for polyomavirus and is associated with clinical improvement in patients with polyomavirus infection.27 CD8+ T cells were found to be increased in mild to moderate COPD lungs and may contribute to inflammation before severe disease.28 Recent findings have identified the presence of polyomavirus infections in COPD patients.29 It is suggested that polyomavirus may have elicited the CD8+ T cell response that led to exacerbation in COPD patients. Polyomavirus has been thought to be closely associated with CD4+ T with B cells,30 and there is an interactive relationship between CD4+ T and CD8+ T,31 as well as CD4+ T driving B cell differentiation. And B cells may be potential transmitters of polyomavirus or involved in the control of polyomavirus.32 CD4+ T can be found to be crucial in the cellular immune response to polyomavirus, and Cao et al. found that CD4+ T is a protective factor against COPD.8 Some findings suggest that polyomavirus infection or persistence may not lead to the development of COPD.33,34 This is inconsistent with the results of the present study, and we believe that the possible reason for this is that all their studies were observational, and there may be errors in the findings.
The varicella zoster virus is left in the body after experiencing chickenpox and reactivates years later in a form called shingles. It can often occur in people whose immune systems are not working properly, such as in respiratory diseases like asthma and COPD.35 For varicella zoster virus, several guidelines have recommended vaccination against varicella zoster virus, and herpes zoster vaccination rates may be low in patients aged 50 years ≥COPD, which may indicate a lack of awareness of herpes zoster virus risk factors among clinicians and patients.36 The results of the present study identified varicella zoster virus as a risk factor for COPD, asthma, and reduced lung function, and therefore varicella zoster virus vaccination may be appropriate for all immunocompromised populations. Importantly, the increased economic burden and morbidity of herpes zoster, its prevention should be done with the use of vaccines, but specific vaccinations are not discussed in this study.
Unlike the present study, risk factors for immune responses to varicella zoster virus-induced immune responses were observed only in asthma. A meta-analysis reports that patients with asthma and COPD have a 24% and 41% increased risk of developing herpes zoster, respectively, compared with healthy controls.37 Asthma and COPD increase the risk of herpes zoster and related complications at any age and may be further elevated in patients receiving inhaled corticosteroids. A Spanish observational, retrospective, non-interventional study found that the most prevalent respiratory diseases (asthma, COPD, and lung cancer) were associated with a higher risk of herpes zoster and postherpetic neuralgia in a standard clinical practice setting.38 The mechanism may be a Th1/Th2 immune imbalance. Similarly, many studies have described an association between asthma and herpes zoster,39 which is consistent with our study. It has been shown that COPD is also a risk factor for herpes zoster virus infection and that the incidence of acute exacerbations of COPD is higher before and after herpes zoster virus attacks.40 However, the results of this study did not find COPD as a risk factor for herpes zoster virus infection.
This study still has some limitations. Firstly, the data for this study were all derived from European populations and further validation with more data from other ethnic populations is still needed to improve the extrapolation of the findings. Second, this study did not find statistical differences in the sensitivity analyses of multiple causal relationships, which may have led to false-positive results in this study. Third, lung diseases usually include both airway and alveolar lesions, and the lack of alveolar-related GWAS data led this study to focus primarily on lung diseases with airway alterations.
ConclusionsThis study provides a comprehensive assessment of the causal relationship between antibody-mediated immune responses phenotypes and COPD, asthma, and lung function, and that the relationship is not confounded by other factors. Seven antibody-mediated immune responses phenotypes that may simultaneously have a causal effect on COPD, asthma, FEV1, and FVC, and anti-polyomavirus 2 IgG seropositivity, and varicella zoster virus glycoproteins E and I antibody levels had a clear role. The results of this study can help to screen people at risk of COPD, asthma, and for early prevention and preemptive diagnosis of pre-COPD, asthma condition.
CRediT Authorship Contribution StatementDrs. LDH and LFR had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: LDH and LFR. Acquisition, analysis, or interpretation of data: XGX, LYL. Drafting of the manuscript: XGX. Statistical analysis: XGX. Obtained funding: LDH and LFR. Supervision: FHY. All authors have read and agreed to the published version of the manuscript.
FundingThis study was funded by the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-D-202003); Natural Science Foundation of China (No: 82374602). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of Competing InterestThe authors declare that they have no competing interests.
AcknowledgementsWe are grateful for the public sharing of GWAS summary data for COPD, asthma, lung function, and antibody-mediated immune responses, which made this work possible.
Data AvailabilityThe data used in this study can be obtained from GWAS Catalog (https://www.ebi.ac.uk/gwas/; antibody-mediated immune responses GWAS ID: GCST90006909 to GCST90006884; COPD: GCST90044074; emphysema: GCST90044073; bronchiectasis: GCST90044075; asthma: GCST90044072; FEV1: GCST90292609; FVC: GCST90292610; FEV1/FVC: GCST90292611), and the code scripts used in this study are available upon reasonable request from the corresponding author.