Herpes zoster (HZ) is a condition that results from the reactivation of the varicella zoster virus (VZV). Several diseases have been reported to increase the risk of developing HZ and postherpetic neuralgia (PHN). The objective of this study is to analyze the prevalence and risk factors for HZ and PHN in the most frequent chronic respiratory diseases, which are chronic obstructive pulmonary disease (COPD), asthma, lung cancer and obstructive sleep apnea (OSA).
MethodsWe conducted an observational, retrospective, non-interventional study between January 2012 and December 2020 based on data from the Castilla-La Mancha Regional Health System in Spain. We used the Savana Manager 3.0 artificial intelligence-enabled system to collect information from electronic medical records.
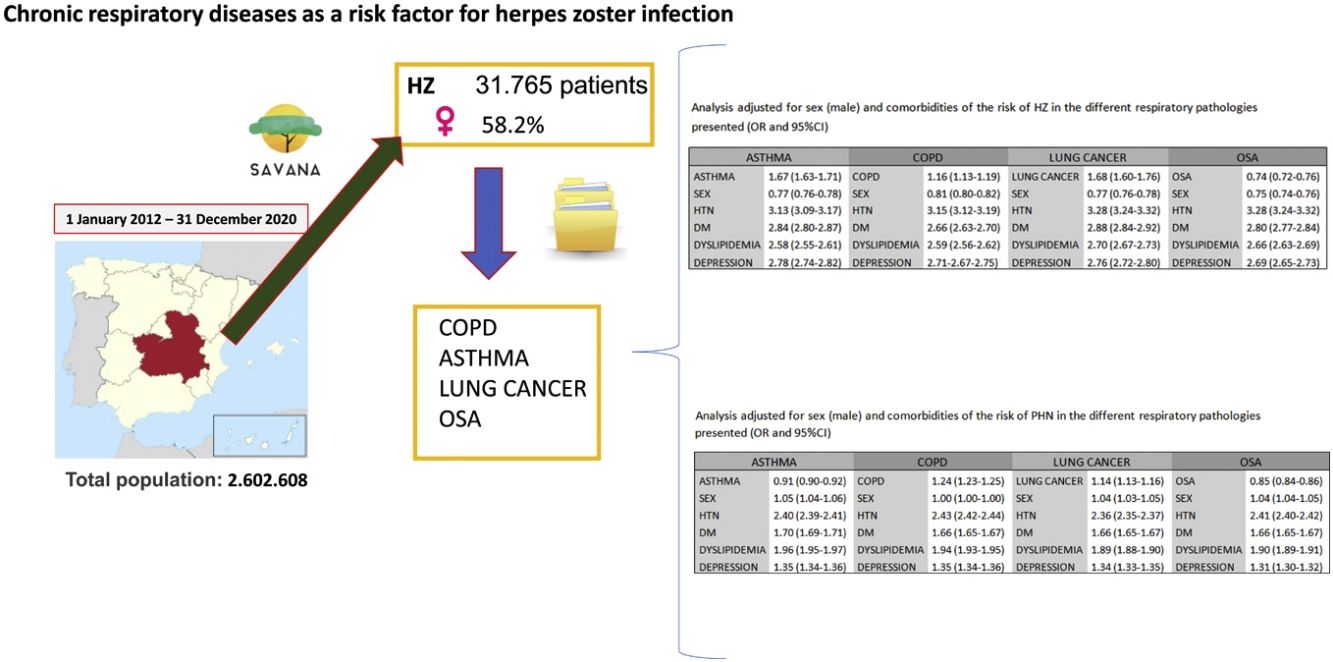
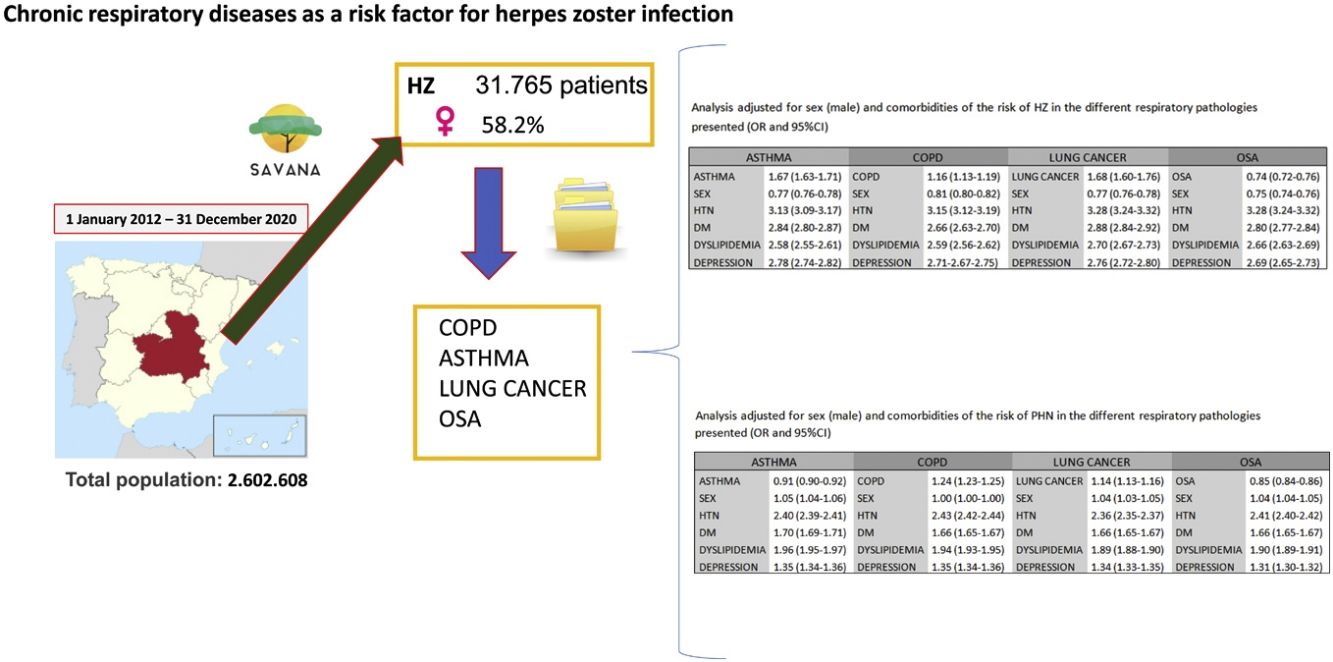
Results31765 subjects presented a diagnosis of HZ. Mean age was 64.5 years (95%CI 64.3–64.7), and 58.2% were women. The prevalence of HZ showed an increasing trend in patients over the age of 50. A risk analysis adjusted for sex and comorbidities in COPD, asthma, lung cancer and OSA presented a higher risk of developing HZ in the first three (OR 1.16 [95%CI 1.13–1.19], 1.67 [1.63–1.71], 1.68 [1.60–1.76], respectively), which further increased in all three when associated with comorbidities. Regarding postherpetic neuralgia, an increased risk was only observed related to COPD and lung cancer (OR 1.24 [95%CI 1.23–1.25], 1.14 [1.13–1.16], respectively), further increasing when associated with comorbidities.
ConclusionsIn a standard clinical practice setting, the most prevalent respiratory diseases (asthma, COPD and lung cancer) are related to a higher risk of HZ and PHN. These data are fundamental to assess the potential impact of vaccination in this population.
Herpes zoster (HZ) is a symptomatic reactivation of the varicella zoster virus (VZV) characterized by a painful vesicular eruption that normally affects a dermatome.1 Primary VZV infection usually occurs in childhood and is manifested by a generalized vesicular eruption. It is estimated that some 90% of adults worldwide may have been infected by VZV,2 while other studies report that 30% may develop HZ due to reactivation of the virus, which remains latent in a dorsal root ganglion.1,2 The natural evolution of HZ entails an acute phase that can last from 2 to 4 weeks and which may become chronic in 5–30% of patients.2
In Europe, the incidence is 3–5 cases per 1000 inhabitants per year.3 The appearance of HZ presents an upwards trend with age, which increases to 60% after the age of 50.4 A suggested explanation for this growing trend is immunosenescence,4,5 which is the progressive age-related decrease in the functionality of the immune system, affecting both innate and adaptive immunity. Women have a higher incidence of HZ compared to men in all age groups.5
The course of HZ involves the appearance of possible complications, including postherpetic neuralgia (PHN),6 which is neuropathic-type pain that can last for months, or even years, and has a significant impact on the quality of life of these patients. The incidence varies from 5% to 15%. The recurrence rate of PHN is around 5%, which can generate a high burden, both for patients as well as healthcare systems.6,7
Many chronic diseases have been considered risk factors for developing HZ, increasing the risk and severity of the disease.7–10 These pathologies include depression, cardiovascular disease, chronic kidney disease, rheumatoid arthritis or diabetes mellitus.7–15 Among the respiratory diseases, chronic obstructive pulmonary disease (COPD),16,17 asthma,18–21 lung cancer22 and obstructive sleep apnea have also been described as risk factors. However, the risk of HZ in patients with these diseases is not entirely well known. There are few published studies, and most are cohort studies that do not always reflect standard clinical practice.
Vaccines are an important tool for the prevention of infections. Since 2006, many countries have been using a live attenuated vaccine in the adult immunocompetent population over 50 years of age as preventive treatment of HZ.23,24 This vaccine has been shown to reduce the appearance of HZ by 61.1% and the incidence of PHN by 66.5%.24 Additionally, it has recently been included in the GOLD recommendations for patients with COPD. The study of large population databases could shed light on this issue and help establish vaccination recommendations in a population group at special risk. It is essential to know these results, since the use of healthcare resources is 17% higher in patients who develop an episode of HZ.24
Our working hypothesis is that leveraging artificial intelligence methodologies on large-scale patient datasets, we can attain a more nuanced understanding of these interrelationships within the context of real-world clinical practice.
The objective of this study is to analyze the real-life risk of developing HZ and its complications in the most prevalent chronic respiratory diseases. With these data, it is possible to identify the risk of HZ and its complications in these patient groups, which may be decisive for the preparation of HZ immunization programs.
Material and methodsParticipantsThe study was performed in the most common chronic respiratory diseases: asthma, COPD, lung cancer and AOS. We have designed an observational, retrospective, non-interventional study using data from the electronic health records of the Castilla-La Mancha Public Health System (SESCAM) in Spain. All patients over 20 years of age who had been diagnosed with HZ were included. Due to the characteristics of HZ infection, we were mostly interested in adult population.
The study period was from January 2012 to December 2020. The total population was 2602608 patients, which had generated a total of 276200601 documents. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were followed for the presentation of observational studies.
Data extraction methodologyArtificial intelligence and Big Data methodology were used with EH READ technology to analyze and the electronic health records (EHR). Savana® Manager is a system that allows unstructured clinical information (free text or natural language) to be obtained from SESCAM EHR. Thanks to this program based on artificial intelligence (natural language processing) and big data techniques, free text is converted into structured and reusable information for research purposes. EHRs used in this study belong to the specialized care network (hospitalization, emergencies, and external consultations) and primary care centers of SESCAM (public health system of Castilla-La Mancha, Spain). The methodology used in the study with the Savana Manager 3.0 platform has been described previously.25–28 Thanks to data processing by the information systems department at each healthcare facility, it is impossible to correlate individual patients with the medical data, and their anonymity is thereby maintained at all times. All information was subsequently validated by computational linguistics (SNOMED CT).29
Information extraction evaluationWhen we evaluated the performance of Savana for correctly identifying mentions of HZ, the results for precision, recall and F-score were 1.0, 0.89 and 0.94, respectively. EHRead also identified PHN with a precision, recall and F-score of 1.0, 0.81 and 0.9, respectively.
Statistical analysisQualitative variables are presented as absolute frequencies and percentages, and quantitative variables as means, 95%CI and standard deviations. Quantitative variables were analyzed using the Student's t-test for independent samples, and the Chi-square test was used for qualitative variables. Differences with a P-value<.05 were considered significant. The OpenEpi version 3.0 (www.OpenEpi.com) and SPSS software (version 25.0; IBM, Armonk, NY, USA) applications were used for the statistical analysis of this study. The adjustment for comorbidities was carried out by fitting a logistic regression to the aggregated data expressed as the number of patients where each of the outcomes, baseline conditions and comorbidities were present. Odds ratios were obtained as the exponential of the logistic coefficients. Calculations were made using Statsmodels in Python 3.9.
Data management and protectionThis study complied with the research practices and regulatory requirements described in the Good Clinical Practice Guideline of the International Council for Harmonization. Likewise, we also followed the latest edition of the Declaration of Helsinki, Guidelines for Good Pharmacoepidemiology Practices, data protection protocols for BigData studies, and local regulations. The study was approved by the Research Ethics Committee of the Guadalajara regional healthcare administration (Ref. CEIm: 2022.27.EO, Approved October 11, 2022). Informed consent was not requested from the patients since, according to European regulations, these documents are not required for retrospective observational studies.
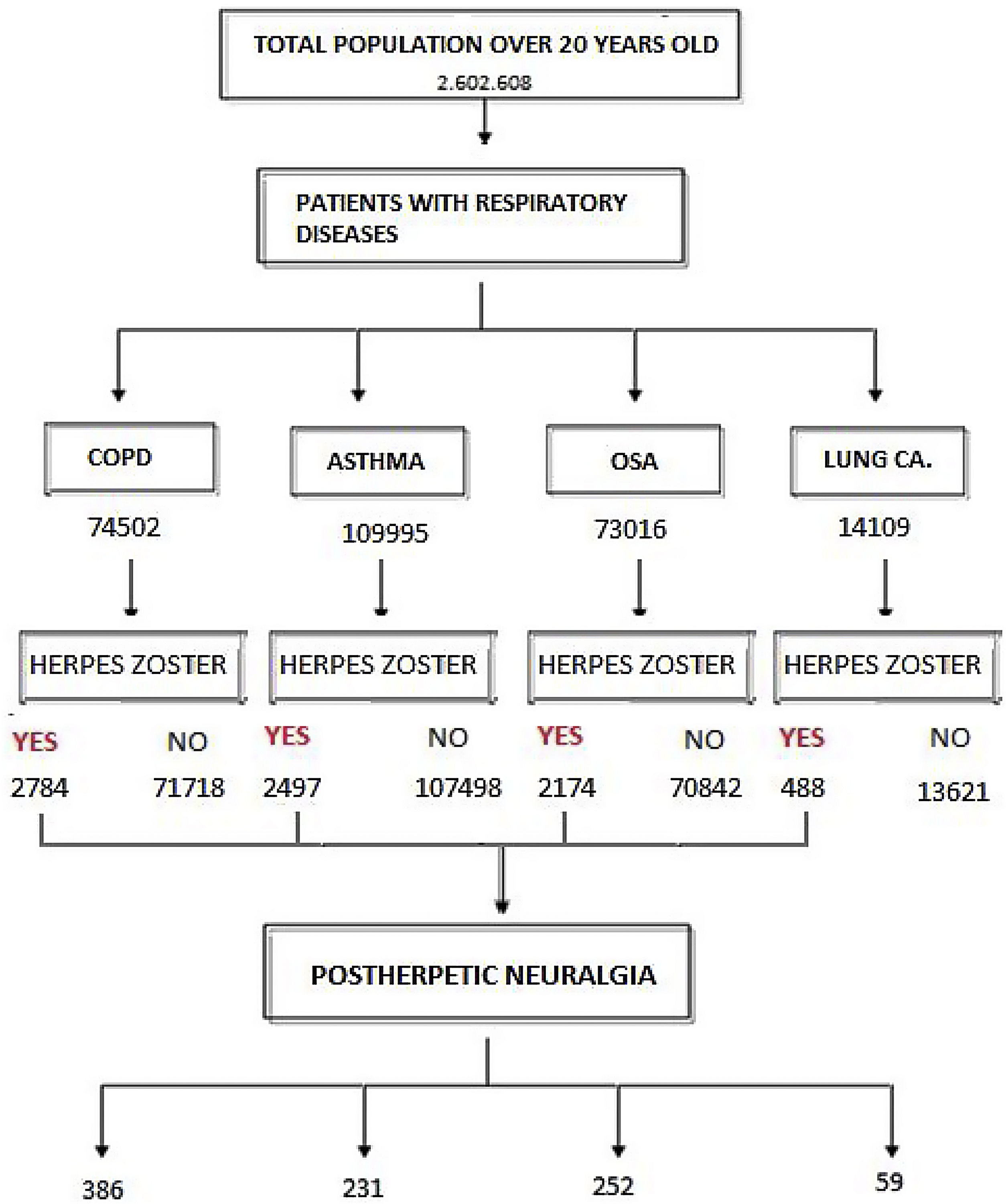
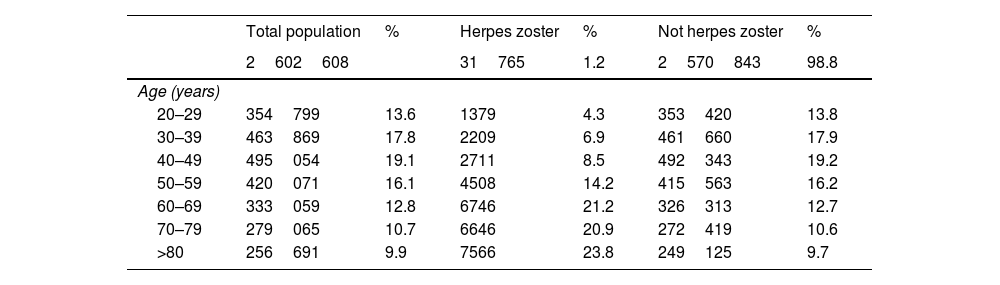
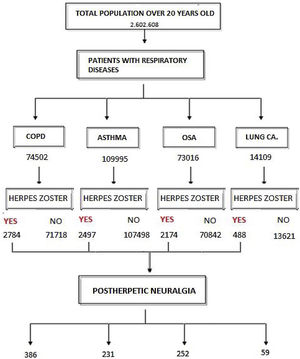
ResultsOut of the total study population over the age of 20 (2602608 patients), HZ infection was diagnosed in 31765 subjects (1.2%). Fig. 1 shows the flowchart of the population with HZ infection in this study: 58.2% were women with a mean age of 64.5 years (95%CI 64.3–64.7), and 41.8% men with a mean age of 64.1 years (95%CI 63.8–64.4) (OR 1.32 [1.29–1.35]). Table 1 shows the distribution by age of subjects older than 20 years, with HZ and without HZ.
Distribution of the general population over 20 years of age, with a diagnosis of HZ and without HZ according to age range.
| Total population | % | Herpes zoster | % | Not herpes zoster | % | |
|---|---|---|---|---|---|---|
| 2602608 | 31765 | 1.2 | 2570843 | 98.8 | ||
| Age (years) | ||||||
| 20–29 | 354799 | 13.6 | 1379 | 4.3 | 353420 | 13.8 |
| 30–39 | 463869 | 17.8 | 2209 | 6.9 | 461660 | 17.9 |
| 40–49 | 495054 | 19.1 | 2711 | 8.5 | 492343 | 19.2 |
| 50–59 | 420071 | 16.1 | 4508 | 14.2 | 415563 | 16.2 |
| 60–69 | 333059 | 12.8 | 6746 | 21.2 | 326313 | 12.7 |
| 70–79 | 279065 | 10.7 | 6646 | 20.9 | 272419 | 10.6 |
| >80 | 256691 | 9.9 | 7566 | 23.8 | 249125 | 9.7 |
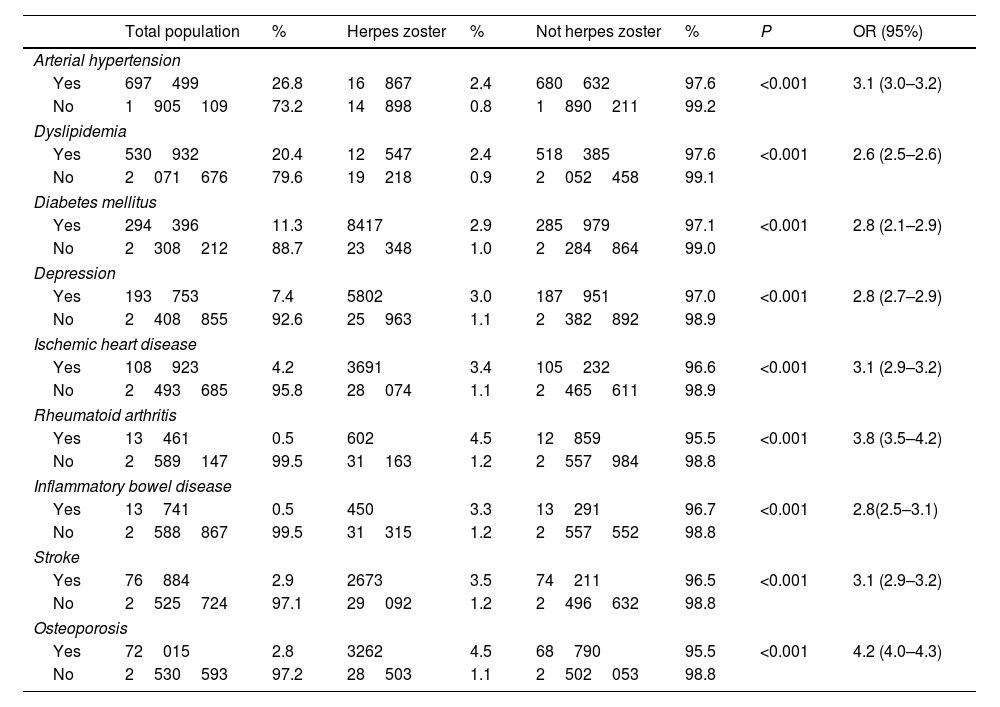
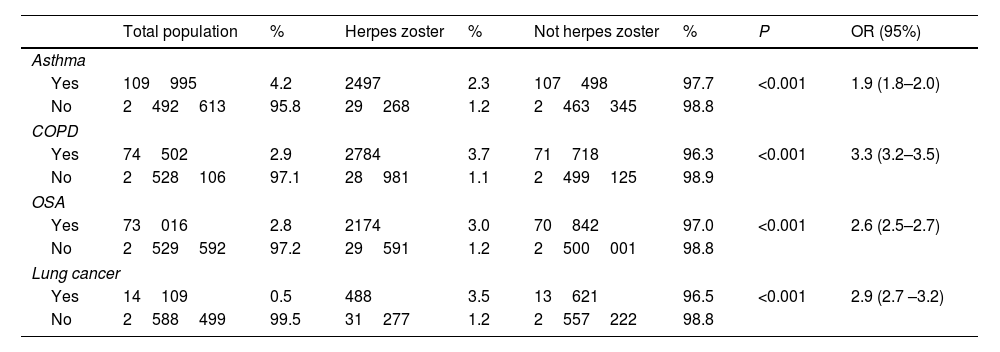
Table 2 shows the prevalence of the main non-respiratory comorbidities identified as risk factors for HZ in the general population with and without HZ. Table 3 shows the incidence of HZ infection in the respiratory diseases studied (COPD, asthma, obstructive sleep apnea [OSA] and lung cancer).
Main non-respiratory comorbidities, defined as risk factors, and their prevalence in the general population over 20 years of age, with and without HZ.
| Total population | % | Herpes zoster | % | Not herpes zoster | % | P | OR (95%) | |
|---|---|---|---|---|---|---|---|---|
| Arterial hypertension | ||||||||
| Yes | 697499 | 26.8 | 16867 | 2.4 | 680632 | 97.6 | <0.001 | 3.1 (3.0–3.2) |
| No | 1905109 | 73.2 | 14898 | 0.8 | 1890211 | 99.2 | ||
| Dyslipidemia | ||||||||
| Yes | 530932 | 20.4 | 12547 | 2.4 | 518385 | 97.6 | <0.001 | 2.6 (2.5–2.6) |
| No | 2071676 | 79.6 | 19218 | 0.9 | 2052458 | 99.1 | ||
| Diabetes mellitus | ||||||||
| Yes | 294396 | 11.3 | 8417 | 2.9 | 285979 | 97.1 | <0.001 | 2.8 (2.1–2.9) |
| No | 2308212 | 88.7 | 23348 | 1.0 | 2284864 | 99.0 | ||
| Depression | ||||||||
| Yes | 193753 | 7.4 | 5802 | 3.0 | 187951 | 97.0 | <0.001 | 2.8 (2.7–2.9) |
| No | 2408855 | 92.6 | 25963 | 1.1 | 2382892 | 98.9 | ||
| Ischemic heart disease | ||||||||
| Yes | 108923 | 4.2 | 3691 | 3.4 | 105232 | 96.6 | <0.001 | 3.1 (2.9–3.2) |
| No | 2493685 | 95.8 | 28074 | 1.1 | 2465611 | 98.9 | ||
| Rheumatoid arthritis | ||||||||
| Yes | 13461 | 0.5 | 602 | 4.5 | 12859 | 95.5 | <0.001 | 3.8 (3.5–4.2) |
| No | 2589147 | 99.5 | 31163 | 1.2 | 2557984 | 98.8 | ||
| Inflammatory bowel disease | ||||||||
| Yes | 13741 | 0.5 | 450 | 3.3 | 13291 | 96.7 | <0.001 | 2.8(2.5–3.1) |
| No | 2588867 | 99.5 | 31315 | 1.2 | 2557552 | 98.8 | ||
| Stroke | ||||||||
| Yes | 76884 | 2.9 | 2673 | 3.5 | 74211 | 96.5 | <0.001 | 3.1 (2.9–3.2) |
| No | 2525724 | 97.1 | 29092 | 1.2 | 2496632 | 98.8 | ||
| Osteoporosis | ||||||||
| Yes | 72015 | 2.8 | 3262 | 4.5 | 68790 | 95.5 | <0.001 | 4.2 (4.0–4.3) |
| No | 2530593 | 97.2 | 28503 | 1.1 | 2502053 | 98.8 | ||
Main respiratory diseases defined as risk factors for HZ and their prevalence in the general population over 20 years of age, with and without HZ.
| Total population | % | Herpes zoster | % | Not herpes zoster | % | P | OR (95%) | |
|---|---|---|---|---|---|---|---|---|
| Asthma | ||||||||
| Yes | 109995 | 4.2 | 2497 | 2.3 | 107498 | 97.7 | <0.001 | 1.9 (1.8–2.0) |
| No | 2492613 | 95.8 | 29268 | 1.2 | 2463345 | 98.8 | ||
| COPD | ||||||||
| Yes | 74502 | 2.9 | 2784 | 3.7 | 71718 | 96.3 | <0.001 | 3.3 (3.2–3.5) |
| No | 2528106 | 97.1 | 28981 | 1.1 | 2499125 | 98.9 | ||
| OSA | ||||||||
| Yes | 73016 | 2.8 | 2174 | 3.0 | 70842 | 97.0 | <0.001 | 2.6 (2.5–2.7) |
| No | 2529592 | 97.2 | 29591 | 1.2 | 2500001 | 98.8 | ||
| Lung cancer | ||||||||
| Yes | 14109 | 0.5 | 488 | 3.5 | 13621 | 96.5 | <0.001 | 2.9 (2.7 –3.2) |
| No | 2588499 | 99.5 | 31277 | 1.2 | 2557222 | 98.8 | ||
Tables s1–s4 of the supplementary material describe the main demographic characteristics of the patients with the respiratory diseases studied and HZ infection, as well as their main associated comorbidities.
Regarding the use of inhaled (IC) and systemic (SC) corticosteroids in patients with asthma, we observed that 79.9% of patients with HZ were regularly treated with IC and 82.6% irregularly with SC. As for COPD patients, 86.6% of patients with HZ received IC as a baseline treatment, and 88.5% were treated with SC very irregularly (mostly sporadically).
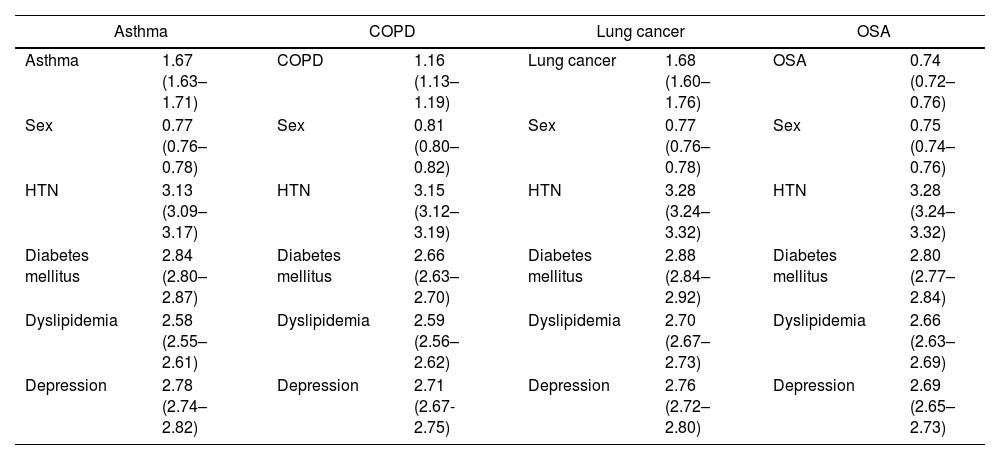
Given the differences associated with sex and the comorbidities that the patients presented, we adjusted the analysis for the risk of HZ associated with the different respiratory diseases analyzed (Table 4). Asthma (OR 1.67 [95%CI 1.63–1.71]), COPD (OR 1.16 [95%CI 1.13–1.19]) and lung cancer (OR 1.68 [95%CI 1.60–1.76]) maintained a risk of developing HZ after adjusting for sex and comorbidities. OSA (OR 0.74 [95%CI 0.72–0.76]) was only associated with developing HZ in the presence of comorbidities.
Analysis adjusted for sex (male) and comorbidities of the risk of HZ in the different respiratory pathologies presented (OR and 95%CI).
| Asthma | COPD | Lung cancer | OSA | ||||
|---|---|---|---|---|---|---|---|
| Asthma | 1.67 (1.63–1.71) | COPD | 1.16 (1.13–1.19) | Lung cancer | 1.68 (1.60–1.76) | OSA | 0.74 (0.72–0.76) |
| Sex | 0.77 (0.76–0.78) | Sex | 0.81 (0.80–0.82) | Sex | 0.77 (0.76–0.78) | Sex | 0.75 (0.74–0.76) |
| HTN | 3.13 (3.09–3.17) | HTN | 3.15 (3.12–3.19) | HTN | 3.28 (3.24–3.32) | HTN | 3.28 (3.24–3.32) |
| Diabetes mellitus | 2.84 (2.80–2.87) | Diabetes mellitus | 2.66 (2.63–2.70) | Diabetes mellitus | 2.88 (2.84–2.92) | Diabetes mellitus | 2.80 (2.77–2.84) |
| Dyslipidemia | 2.58 (2.55–2.61) | Dyslipidemia | 2.59 (2.56–2.62) | Dyslipidemia | 2.70 (2.67–2.73) | Dyslipidemia | 2.66 (2.63–2.69) |
| Depression | 2.78 (2.74–2.82) | Depression | 2.71 (2.67-2.75) | Depression | 2.76 (2.72–2.80) | Depression | 2.69 (2.65–2.73) |
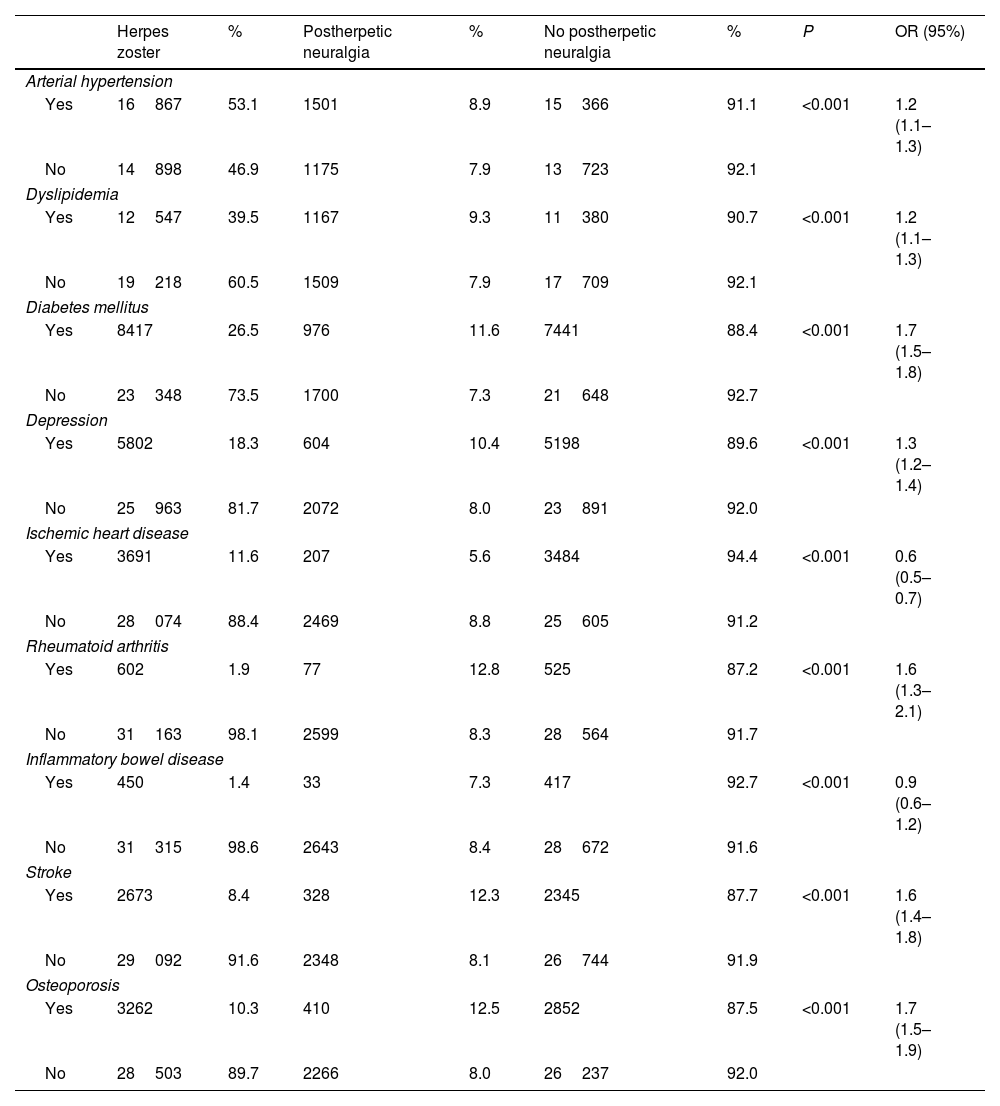
Among the patients who presented HZ, the population that experienced PHN was analyzed (8.4%; 2676 subjects). Among them, 56.9% were women with a mean age of 73.5 years (95%CI 72.8–74.2), and 43.1% were men with a mean age of 73.4 years (95%CI 72.6–74.1) (OR 0.9 [0.8–1.0]). Table 5 shows the main non-respiratory comorbidities in patients with HZ and PHN. Table 6 analyzes the main respiratory diseases in this population.
Main non-respiratory comorbidities, defined as risk factors, and their prevalence in the population with herpes zoster, with and without PHN.
| Herpes zoster | % | Postherpetic neuralgia | % | No postherpetic neuralgia | % | P | OR (95%) | |
|---|---|---|---|---|---|---|---|---|
| Arterial hypertension | ||||||||
| Yes | 16867 | 53.1 | 1501 | 8.9 | 15366 | 91.1 | <0.001 | 1.2 (1.1–1.3) |
| No | 14898 | 46.9 | 1175 | 7.9 | 13723 | 92.1 | ||
| Dyslipidemia | ||||||||
| Yes | 12547 | 39.5 | 1167 | 9.3 | 11380 | 90.7 | <0.001 | 1.2 (1.1–1.3) |
| No | 19218 | 60.5 | 1509 | 7.9 | 17709 | 92.1 | ||
| Diabetes mellitus | ||||||||
| Yes | 8417 | 26.5 | 976 | 11.6 | 7441 | 88.4 | <0.001 | 1.7 (1.5–1.8) |
| No | 23348 | 73.5 | 1700 | 7.3 | 21648 | 92.7 | ||
| Depression | ||||||||
| Yes | 5802 | 18.3 | 604 | 10.4 | 5198 | 89.6 | <0.001 | 1.3 (1.2–1.4) |
| No | 25963 | 81.7 | 2072 | 8.0 | 23891 | 92.0 | ||
| Ischemic heart disease | ||||||||
| Yes | 3691 | 11.6 | 207 | 5.6 | 3484 | 94.4 | <0.001 | 0.6 (0.5–0.7) |
| No | 28074 | 88.4 | 2469 | 8.8 | 25605 | 91.2 | ||
| Rheumatoid arthritis | ||||||||
| Yes | 602 | 1.9 | 77 | 12.8 | 525 | 87.2 | <0.001 | 1.6 (1.3–2.1) |
| No | 31163 | 98.1 | 2599 | 8.3 | 28564 | 91.7 | ||
| Inflammatory bowel disease | ||||||||
| Yes | 450 | 1.4 | 33 | 7.3 | 417 | 92.7 | <0.001 | 0.9 (0.6–1.2) |
| No | 31315 | 98.6 | 2643 | 8.4 | 28672 | 91.6 | ||
| Stroke | ||||||||
| Yes | 2673 | 8.4 | 328 | 12.3 | 2345 | 87.7 | <0.001 | 1.6 (1.4–1.8) |
| No | 29092 | 91.6 | 2348 | 8.1 | 26744 | 91.9 | ||
| Osteoporosis | ||||||||
| Yes | 3262 | 10.3 | 410 | 12.5 | 2852 | 87.5 | <0.001 | 1.7 (1.5–1.9) |
| No | 28503 | 89.7 | 2266 | 8.0 | 26237 | 92.0 | ||
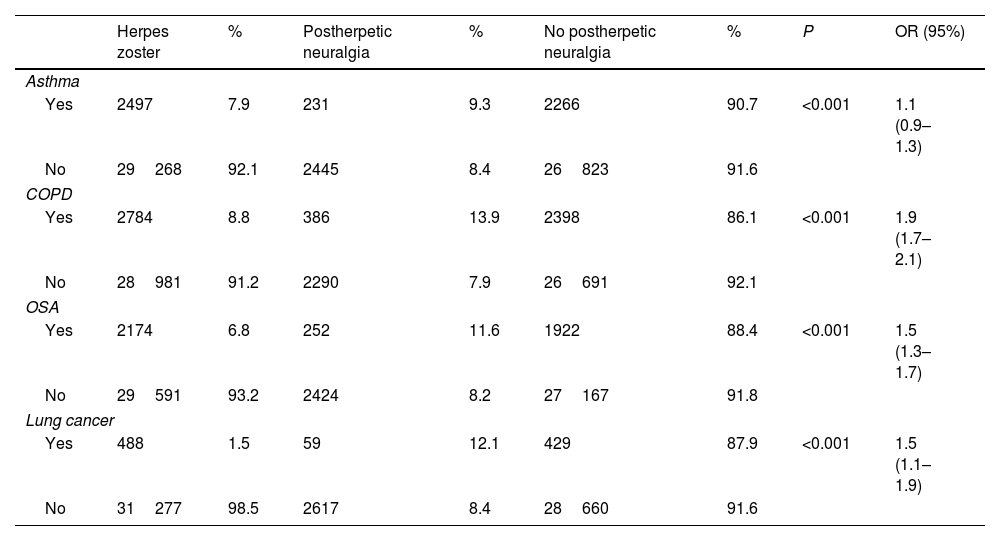
Main respiratory pathologies and their prevalence in the population with herpes zoster, with and without PHN.
| Herpes zoster | % | Postherpetic neuralgia | % | No postherpetic neuralgia | % | P | OR (95%) | |
|---|---|---|---|---|---|---|---|---|
| Asthma | ||||||||
| Yes | 2497 | 7.9 | 231 | 9.3 | 2266 | 90.7 | <0.001 | 1.1 (0.9–1.3) |
| No | 29268 | 92.1 | 2445 | 8.4 | 26823 | 91.6 | ||
| COPD | ||||||||
| Yes | 2784 | 8.8 | 386 | 13.9 | 2398 | 86.1 | <0.001 | 1.9 (1.7–2.1) |
| No | 28981 | 91.2 | 2290 | 7.9 | 26691 | 92.1 | ||
| OSA | ||||||||
| Yes | 2174 | 6.8 | 252 | 11.6 | 1922 | 88.4 | <0.001 | 1.5 (1.3–1.7) |
| No | 29591 | 93.2 | 2424 | 8.2 | 27167 | 91.8 | ||
| Lung cancer | ||||||||
| Yes | 488 | 1.5 | 59 | 12.1 | 429 | 87.9 | <0.001 | 1.5 (1.1–1.9) |
| No | 31277 | 98.5 | 2617 | 8.4 | 28660 | 91.6 | ||
In supplementary material, Tables s5–s8 show the analysis by sex, age and comorbidities in patients with respiratory diseases and PHN.
In patients with asthma or COPD and PHN, the use of IC and SC was also analyzed, showing that 86.1% of asthmatic patients habitually used IC, while 87% had taken SC at some point. Meanwhile, 90.4% of patients with COPD had used IC regularly, and 90.9% SC irregularly. Table 7 describes an analysis adjusted for comorbidities and sex in patients with respiratory disease and PHN.
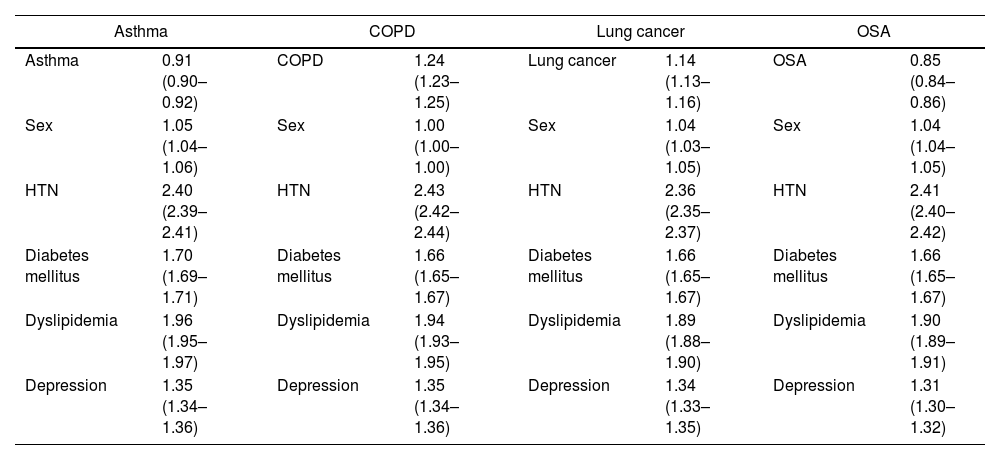
Analysis adjusted for sex (male) and comorbidities of the risk of PHN in the different respiratory pathologies presented (OR and 95%CI).
| Asthma | COPD | Lung cancer | OSA | ||||
|---|---|---|---|---|---|---|---|
| Asthma | 0.91 (0.90–0.92) | COPD | 1.24 (1.23–1.25) | Lung cancer | 1.14 (1.13–1.16) | OSA | 0.85 (0.84–0.86) |
| Sex | 1.05 (1.04–1.06) | Sex | 1.00 (1.00–1.00) | Sex | 1.04 (1.03–1.05) | Sex | 1.04 (1.04–1.05) |
| HTN | 2.40 (2.39–2.41) | HTN | 2.43 (2.42–2.44) | HTN | 2.36 (2.35–2.37) | HTN | 2.41 (2.40–2.42) |
| Diabetes mellitus | 1.70 (1.69–1.71) | Diabetes mellitus | 1.66 (1.65–1.67) | Diabetes mellitus | 1.66 (1.65–1.67) | Diabetes mellitus | 1.66 (1.65–1.67) |
| Dyslipidemia | 1.96 (1.95–1.97) | Dyslipidemia | 1.94 (1.93–1.95) | Dyslipidemia | 1.89 (1.88–1.90) | Dyslipidemia | 1.90 (1.89–1.91) |
| Depression | 1.35 (1.34–1.36) | Depression | 1.35 (1.34–1.36) | Depression | 1.34 (1.33–1.35) | Depression | 1.31 (1.30–1.32) |
Using artificial intelligence, we have analyzed patients with different respiratory diseases to determine their risk for HZ and secondary PHN under conditions of normal clinical practice. The study was conducted in a large population over a 9-year period.
HZ was present in 1.2% of the general population over the age of 20 years. The mean age of patients with HZ was 64 years, with a predominance of females. There is still no definitive justification for this predominance in the female sex, but proposed explanations include hormonal differences or the greater longevity of women versus men.5,30 A growing trend of HZ is observed as patients age, which accelerates after the age of 50. The prevalence of HZ is higher in the population over 60 years of age, reaching a maximum in the >80 age group. The vast majority of published studies31 analyze study populations over 65 years of age and do not include the under-50 population. Our study includes a younger population (>20 years of age), which did not have an increased risk of developing HZ. Our results support the idea that immunosenescence may play a role in the development of HZ.4,5 The varicella zoster virus remains latent in the dorsal root ganglia thanks to cell-mediated immunity (CD4 and CD8 T lymphocytes, and memory T cells). When this immunity decreases, the reactivation of the VZV would take place over time, leading to the development of HZ. This point is noteworthy since it is estimated that the world population over 65 years of age will be 30.4% by the year 2050.4
However, not only aging or being female seems to be associated with increased risk of HZ. Our results confirm that patients with certain comorbidities, such as rheumatoid arthritis, depression, arterial hypertension or osteoporosis, present a higher risk of developing HZ compared to the general population without these comorbidities. These results are consistent with previously published reports.8–15,31
The respiratory diseases analyzed in the study also presented an increased risk for HZ. Patients with a diagnosis of asthma presented 1.9 times more risk for HZ than patients without asthma. In this group, the majority of patients were females. The risk was especially high in the population over 50 years of age, reaching its maximum in the population over 70 years of age. These patients presented more comorbidities than the general population. In an analysis adjusted for comorbidities and sex, asthma continued to be an independent risk factor for HZ, which increased in the presence of associated comorbidities. These results are consistent with the literature, where a positive association has been found between asthma and the risk of HZ in the adult population. A 4.1-times greater risk of HZ has been described in patients from 41 to 60 years of age, which increases up to 23-times higher risk in the population over 60 years of age.18–20 Although several mechanisms have been proposed, the specific factors of this relationship are unknown.20,32
COPD has also been described as a risk factor for HZ.16,17 It has been suggested that the reduced activity of macrophages, natural killer cells or polymorphonuclear cells in COPD patients may have a possible relationship with the increased incidence of HZ in these patients.16 Our population presented a 3.3-times higher risk of HZ, with a predominance of males and an increased risk in patients over the age of 70. In a subsequent adjusted analysis, COPD remained an independent risk factor for HZ, with an OR of 1.16, which increased with the presence of associated comorbidities. Muñoz et al.33 analyzed a population over 50 years of age for 6 years and described a 45% increased risk of presenting HZ in the COPD population compared to the non-COPD population.
In the literature, an increased HZ risk of 61% has been described in COPD patients with inhaled corticosteroids, which reaches up to 90% in patients with asthma who use inhaled corticosteroids.34 However, given the widespread use of IC in COPD and asthma and the great variability in the use of SC, it was not possible to correctly analyze the impact of these treatments in these 2 diseases.
Regarding lung cancer as a risk factor for developing HZ, our population presented 2.9-times more risk than the population without lung cancer. This population demonstrated greater risk at ages over 60 years and when associated with comorbidities. In a subsequent adjusted analysis, lung cancer alone presented 1.68 times more risk for HZ, which increased with the presence of comorbidities. Recent studies have described an increased risk of HZ in patients with solid and hematological tumors, which was higher in those receiving chemotherapy.35 A greater risk of HZ has also been described in patients with lung cancer undergoing treatment with immunotherapy.36 However, said cohort studies collected a low number of patients, and ours is the first to collect a large population base that exclusively describes lung cancer as a risk factor for HZ. In this study, it was not possible to carry out a differential analysis between small cell and non-small cell lung cancer, nor the impact of the various treatments that the patients received.
Patients with obstructive sleep apnea presented a 2.6-times higher risk of HZ than patients without OSA, although this risk disappeared in a subsequent analysis adjusted for comorbidities, occurring only in those with associated comorbidities. In fact, a significant lower OR may suggest that OSA may be a protective factor. This relationship should be further analyzed in a specific study of this pathology. We found only one cohort article in the literature that studied whether these patients have a higher risk of HZ. This study was conducted in an Asian population and showed a risk that was 1.23-times greater than the non-OAS population.37 Our study is the first to analyze a European population, adjusted for gender and different comorbidities.
When we analyzed the risk of PHN, the incidence was similar to reports in the literature, mostly affecting women.38,39 The age of onset was over 70 years, which was also similar to previous studies.6 Non-respiratory comorbidities that had a higher risk of PHN were analyzed, and arterial hypertension, diabetes mellitus, dyslipidemia, depression, rheumatoid arthritis, stroke and osteoporosis were shown to be the most related. These data are similar to reports in the literature6,7. However in our population, those patients who suffered ischemic heart disease and inflammatory bowel disease had a lower risk of PHN than those who did not present these comorbidities, differing from previous literature. Maybe HZ infection and PHN have different risk factor and pathogenic mechanisms. These results must open the door for future research in prospective studies.
We also specifically analyzed the most prevalent respiratory diseases. Patients with asthma and HZ had a 1.1-times higher risk of PHN, again with a predominance of women. As for age, those over the age of 70 most frequently presented this complication. The risk increased when associated with comorbidities, such as hypertension, diabetes mellitus, dyslipidemia, or depression. However, in a subsequent analysis adjusted for sex and comorbidities, the diagnosis of asthma did not maintain a higher risk of developing PHN, and it was the male sex that presented the highest risk, which increased with the presence of comorbidities. These results differ from the previous literature, since some studies have described an increased risk of PHN in asthmatic patients of up to 1.21,34 the majority being women.
COPD presented 1.9 times more risk of PHN, being more frequent in men over 70 years of age. As in asthma, the presence of comorbidities increased the risk of PHN. In a subsequent analysis adjusted for sex and comorbidities, the risk was 1.24, which increased with the presence of arterial hypertension, dyslipidemia, diabetes and depression. The male sex was maintained as a risk factor for developing PHN. In a study carried out over a 6-year period, Muñoz et al.33 described a 1.88-times greater risk of PHN in COPD patients.
The use of inhaled corticosteroids in patients with asthma or COPD and with PHN was greater than 85%. The use of systemic corticosteroids was very irregular over time, and it is therefore difficult to establish a causal relationship.
Regarding lung cancer, the risk of PHN was 1.5 times greater than in the non-cancer population, mainly affecting men over the age of 70. In the subsequent adjustment for sex and comorbidities, the independent risk of developing PHN in patients with lung cancer was 1.14, predominantly in males and increasing with the presence of associated diseases. Previous studies have analyzed the relationship of certain types of cancer, such as hepatic or hematological, with the increased development of PHN, finding no evidence of increased risk.35 In our review of the literature, we have found no studies specifically directed at patients with lung cancer and its possible relationship with the risk of developing PHN, and our group is the first to be able to shed light on this subject.
Lastly, we analyzed the relationship between OSA and PHN and found a 1.5-times higher risk; however, when the analysis was adjusted for comorbidities, no correlation was observed. In addition, we have found no studies in the literature analyzing the possible relationship between these 2 pathologies.
The main strength of this study is the large population analyzed under conditions of normal clinical practice, and it is the first to analyze – using artificial intelligence – the development of HZ at a population level together with some of the most prevalent respiratory diseases. This minimizes the possible selection biases that can be found in previously published studies, the vast majority of which are cohort studies with small populations. Through this approach, we provide an overview that is essential to understand the true magnitude of this problem. This knowledge serves as a critical guide for the informed development of both prevention and treatment strategies, including vaccination programs.
This has been made possible by the complete digitalization of clinical information in the Castilla-La Mancha regional public healthcare system since 2012. The main limitation is the potential lack of information registered in the electronic health records. Therefore, our study only focuses on variables with verified information quality. Other limitation could be the lack of representativeness (the study was made only in one part of Spain). Due to our public health system no large differences are expected in several regions of Spain. Our large study population of more than 2 million subjects can extrapolate our results to countries with similar patient characteristics and similar health systems.
ConclusionsThis study identifies the main risk factors for HZ, specifically focusing on the most prevalent respiratory diseases. Using artificial intelligence (PLN) techniques, we can determine the characteristics of patients at higher risk of developing HZ and PHN in real life, which can be used as a reference when implementing specific prevention plans, including vaccination against HZ.
Authors’ contributionsConceptualization, DM, JMR and JLI; methodology, SM and DM; software, MB and GL; validation, DM, MC, CC; investigation, DM; composition/original draft preparation, DM; composition/revision and editing, DM and JLI; visualization, DM; supervision, JLI. All authors have read and agreed to the published version of the manuscript.
FundingSupported by the Chair of Inflammatory Diseases of the Airways, University of Alcalá (Alcalá de Henares, Spain).
Conflict of interestsJL Izquierdo received professional fees from ASTRA ZENECA, BAYER, BOEHRINGER INGELHEIM, CHIESI, GSK, GRIFOLS, MENARINI, NOVARTIS, ORION, PFIZER, SANDOZ, TEVA and Zambon during the study.
José Miguel Rodríguez received professional fees from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, FAES, Grifols, Menarini, Novartis, Orion, Pfizer, Roche y Teva.
Diego Morena, Carolina Campos, María Castillo, Sara Lumbreras and María Benavent have no conflicts of interest to declare regarding this study.