In recent years, personalized or precision medicine has made effective inroads into the management of diseases, including respiratory diseases. The route to implementing this approach must invariably start with the identification and validation of biological biomarkers that are closely related to the diagnosis, treatment, and prognosis of respiratory patients. In this respect, biological biomarkers of greater or lesser reliability have been identified for most respiratory diseases and disease classes, and a large number of studies are being conducted in the search for new indicators. The aim of this review is to update the reader and to analyze the existing scientific literature on the existence and diagnostic, therapeutic, and prognostic validity of the most important biological biomarkers in the main respiratory diseases, and to identify future challenges in this area.
En los últimos años la llamada «medicina personalizada o de precisión» ha irrumpido con fuerza en el manejo de las enfermedades, entre ellas las respiratorias. La posibilidad de implantar esta forma de trabajar pasa indefectiblemente por el hallazgo y validación de biomarcadores biológicos que se relacionen bien con el diagnóstico, tratamiento o pronóstico de los pacientes respiratorios. En este sentido, la mayoría de enfermedades respiratorias o grupo de las mismas ya cuentan con biomarcadores biológicos de mayor o menor fiabilidad, y se están realizando un gran número de estudios en busca de nuevos de estos indicadores. El objetivo de la presente revisión es poner al día al lector y analizar la literatura científica existente sobre la existencia y validez diagnóstica, terapéutica o pronóstica de los biomarcadores biológicos más importantes en la actualidad en las principales enfermedades respiratorias, así como sobre los retos futuros en este sentido.
Biomarkers, defined as a measurable characteristic that constitutes an indicator of a normal or pathogenic biological process or response to an exposure or intervention,1,2 have acquired a key role in respiratory medicine in the development of a patient-based, as opposed to disease-based, therapeutic approach.3 Biomarkers need to have high specificity for the disease or event to be evaluated, they must be easy and inexpensive to measure, show good discriminative capacity, and be more cost effective than indicators currently used in conventional clinical practice.4,5
Biomarkers are generally classified according to their field of application or their nature.4,5 In terms of their application, they are defined as diagnostic, monitoring, pharmacodynamic/response, predictive, prognostic, safety, and susceptibility/risk markers.4
Although imaging or pulmonary function tests can provide proven respiratory biomarkers, most have been developed from “omic-” based procedures, giving rise to genomic, transcriptomic, proteomic, metabolomic and epigenetic biomarkers.6–9 However, digital biomarkers collected from electronic devices10 that provide continuous, real-time information on complex parameters related to respiratory health must also be taken into consideration.
The expansion of biomarkers and their growing importance in the management of respiratory diseases has prompted us to critically evaluate the characteristics, conditions and applicability of the main biomarkers currently available for the management of patients with respiratory diseases.
Biomarkers in chronic obstructive pulmonary diseaseChronic obstructive pulmonary disease (COPD) is a condition associated with considerable morbidity and mortality.11 The heterogeneous nature of this disease makes it difficult for clinicians to predict prognosis and response to treatment exclusively on the basis of clinical or functional data. For this reason, the search for diagnostic, prognostic and treatment response biomarkers in COPD has been one of the most important and fruitful fields of research in recent decades.
In the stable phase, biomarkers associated with interleukin (IL)-6-mediated inflammation, such as C-reactive protein (CRP) and fibrinogen, are known to be associated with an increased risk of death from COPD,12 and also define a pattern of increased risk of moderate and severe exacerbations.13 Studies investigating other biomarkers have shown that CC16 (club cell protein 16) and sRAGE (soluble receptor for advanced glycation end products) are associated with decreased lung function and emphysema progression, although the association is somewhat weak.14–16
Blood levels of miR-320c, which inhibits SERPINA1 expression in liver cells, are associated with the presence of lung disease in patients with different serum levels of alpha-1 antitrypsin.17 There is also evidence that interstitial expression of SOD3 and fibulin-5 in COPD patients is diminished, and that methylated miR-7 levels are elevated in patients with emphysema.18,19
The most interesting development is probably the use of peripheral blood eosinophil counts as a biomarker of response to treatment with inhaled corticosteroids (ICS). Eosinophil counts of over 300cells/mm3 indicate that adding an ICS to the patient's treatment will reduce the risk of COPD exacerbation. This biomarker has been extensively studied in population samples and clinical trials,20–26 and is a first step towards precision medicine in COPD.27,28
Biomarkers in asthmaAsthma, like COPD, is also a heterogeneous syndrome29 with a broad pheno-endotypic spectrum involving many different mediators. This variability makes it difficult to pinpoint a single biomarker that can help predict severity, evolution, and response to treatment.
Inflammatory mediators of asthma can be measured in various body samples, including the upper and lower airway, saliva, urine, and peripheral blood,30 although each type of sample has its advantages and limitations (Table 1).31
Advantages and limitations of different biomarker sampling methods in asthma.
| Methods | Biomarker | Cut-off point | Advantages | Limitations |
|---|---|---|---|---|
| Bronchoscopy:-Biopsy-Broncho-alveolar lavage (BAL)-Bronchial brushing | - Eosinophils- Neutrophils- Total inflammatory cell counts- Cytokines- Leakage of markers and mediators- Airway remodeling | No clear cut-off points | Semi-direct read-out | - Invasive- Requires expert staff- Not feasible in very severe disease with compromised lung function- Potential sampling site bias- Dilution (BAL) |
| Induced sputum | - Eosinophils- Neutrophils- Total inflammatory cell count- Cytokines- Cell activation markers- No mediators | In general, a cut-off point of ≥3% is used to indicate sputum eosinophilia, and ≥61% to indicate sputum neutrophilia.However, adapting treatment on the basis of sputum eosinophils has established various sputum eosinophil cutoffs, ranging from 2% to 8%. | - Semi-direct read-out- Multiple biomarkers- Reproducible read-out- Appropriate method for disease phenotyping and follow-up in specialized centers | - Semi-invasive- Analyzable samples obtained from approx. 80%––90% of subjects- Adapted protocol needed for very severe disease with compromised lung function(contraindicated if FEV1<1L and/or with concomitant heart disease- Technically complex, time-consuming procedure, restricted to specialized centers |
| Peripheral blood | - Eosinophils- Cell activation markers- IgE (total/specific)- Cytokines andmediators | Various cut-off values, mostly ranging 150–500cells/μL are used for blood eosinophils | Easy to collect | - Semi-invasive- Indirect read-out- High intra-subject diurnal variability- Blood eosinophils do not adequately reflect airway eosinophilia during treatment with systemic corticosteroids |
| Exhaled breath | - FeNO-Volatile organic compounds (VOC) | Low:FeNO<25 ppb (≥12 years), <20 (<12 years), high FeNO> 50 (≥12 years), <35 (<12 years) | - Non-invasive- Simple method that allows repeat measurements- Appropriate method for phenotyping and monitoring- Direct read-out | - Various factors alter FeNO levels- No standardized VOC collection and analysis methods |
| Exhaled breath condensate | - pH- Markers of oxidative stress- Leukotrienes- Cytokines | No clear cut-off points.Some studies show that pH ≤7.20 is related to poorly controlled asthma | - Non-invasive- Allows serial measurements | - Requires a specialized laboratory- Expensive- Variable results due to technical issues- Requires further developmentand validation |
Taken and adapted from: Diamant, et al.31.
Various molecular mechanisms related to asthma clinical phenotypes, particularly T2 asthma, have recently been identified.32 Sputum eosinophils is probably the best characterized and most useful T2 asthma biomarker identified so far. The analysis of induced sputum to determine central airway inflammation is a reproducible sampling method that is less invasive than bronchoscopy; however, it is time-consuming and must be performed in a specialized center.33 The usefulness of other biomarkers, such as immunoglobulin E (IgE), blood eosinophils, measurement of fractional exhaled nitric oxide (FeNO), or periostin, is still unclear.34,35 Therefore, although serum eosinophils do not always correlate with sputum eosinophils,36 they predict response to anti-IL5-IL-5Rα5 biologics,30 and several studies appear to support their usefulness as a predictor of exacerbations.37,38 There is also solid evidence of the association between serum vitamin D levels, asthma control, and the incidence of exacerbations.39 Serum IgE is used to decide the omalizumab regimen, but does not predict therapeutic response.40 FeNO is associated with eosinophilic airway inflammation, which can help diagnose asthma41 and could even identify dupilumab responders.42
Studies in other identifiable serum markers, such as CD26 (a marker of T cell activation), or CD14 (a monocyte-associated marker), insulin-like growth factors, or identifiable factors in exhaled breath condensate, such as mitochondrial or nuclear DNA, suggest they may be useful in establishing asthma pheno-endotypes, since they appear to be capable of differentiating allergic from non-allergic asthma, and have been associated with asthma severity and airway remodeling.43–47
These mediators in isolation do not fulfill the criteria for an ideal biomarker, so the use of combined panels will probably improve the identification of asthma endotypes.
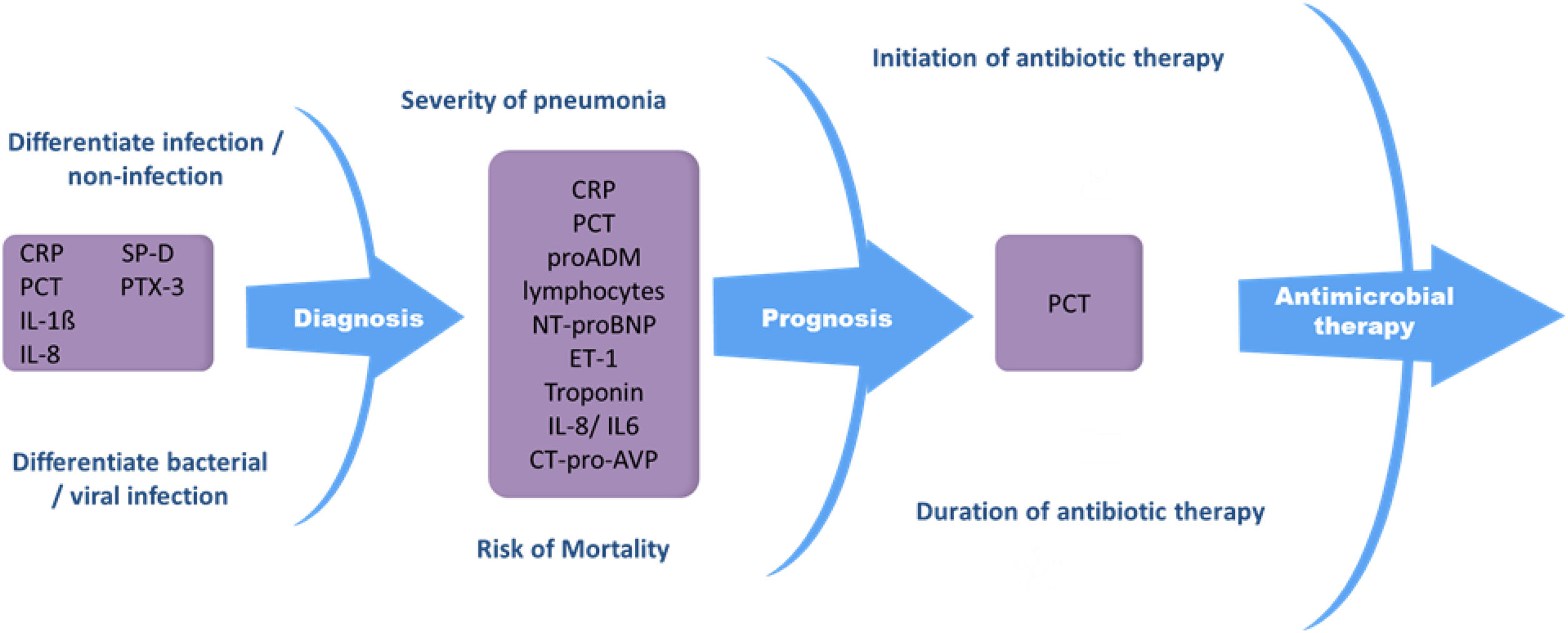
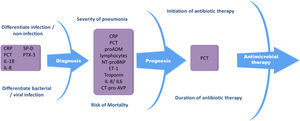
Biomarkers in pneumoniaBiomarkers can be used in both the diagnosis48 and treatment of community-acquired and nosocomial pneumonia, since they help differentiate between bacterial and viral infection,49–56 identify and stratify patients with severe pneumonia,57–62 identify pneumonia-related complications,63,64 and indicate when to start and end antibiotic treatment53,65–69 (Fig. 1). Biomarkers provide reliable information about host response to infection as well as pathogenic activity within the host. These factors can support clinical parameters and aid in decision-making.
Procalcitonin and CRP are still the most commonly used biomarkers in pneumonia.52,67,70–72 Other biomarkers, such as proadrenomedullin (pro-ADM),73,74 IL-6,75–77 IL-8, N-terminal pro b-type natriuretic peptide (NT-proBNP),61,78,79 C-terminal portion of pro-arginine-vasopressin (CT-pro-AVP),80 pentraxin 3 (PTX3),81,82 fibroblast growth factor 21 (FGF21),83 serum amyloid A (SAA)78 and surfactant protein D (SP-D)84 have recently been evaluated, although further studies are needed to determine their role as pneumonia markers. Biomarker levels in pneumonia can vary considerably, since they can be influenced by factors such as immune status, immunomodulatory therapy, the pathogen itself, disease severity, and the timing of biomarker determination with respect to the start of infection.85 This is one of their main drawbacks in clinical practice. There is a high level of evidence that biomarkers such as CRP and PCT should be considered decision support tools, and that they are most useful when used together with clinical parameters and severity scoring systems.86–89 Despite the many challenges yet to be surmounted in biomarker research, these parameters can substantially improve the management of patients with pneumonia.
Biomarkers in bronchiectasis and cystic fibrosisThe clinical and biological manifestations of cystic fibrosis and bronchiectasis are highly heterogeneous and complex due to the different pathophysiological mechanisms that determine their severity and prognosis, hence the importance of biomarkers that can identify clinical phenotypes and molecular endotypes in these patients, making it possible to administer personalized and targeted treatments.90,91
For many years, sputum color, which reflects pulmonary inflammation, mainly neutrophilic, has been the most widely used and affordable biomarker of poor prognosis in these patients.92 Nowadays, however, major advances in research have led to the identification of quantifiable blood-based and lung biomarkers that play a diagnostic, prognostic and even therapeutic role (Table 2).
Main biomarkers in bronchiectasis and cystic fibrosis and detection method.
| Category | Biomarkers | Detection method |
|---|---|---|
| Proteases | Neutrophil elastase | ELISA, semi-quantitative ELISA neutrophil elastase airway test stick – NEAT stick© |
| Metalloproteases | ELISA | |
| Mucins | MUC5AC and MUC5AB | ELISA, chromatography |
| Antimicrobial proteins and peptides | LL-37, SLPI, Lactoferrin, Lysozyme | ELISA |
| Microbiology | Lung bacterial load | Microbiological culture (semi-quantitative), qPCR (quantitative) |
| Pulmonary dysbiosis | Microbiome (16s RNA) | |
| Systemic inflammation | White blood cells, neutrophils, platelets and erythrocyte sedimentation rate | Complete blood count, flow cytometry |
| CRP, TNF-α | ELISA |
CRP: C-reactive protein; ELISA: enzyme-linked immunosorbent assay; qPCR: quantitative polymerase chain reaction; SLPI: leukocyte protease inhibitor; TNF-α: tumor necrosis factor alpha.
Lung proteases have been the most widely studied biomarkers in both diseases due to their key role in perpetuating inflammation and lung damage in these patients.93 One of the most important is neutrophil elastase, which has been shown to be a powerful marker of prognosis and severity and a good therapeutic target in patients with bronchiectasis.94,95 It is important to note that the biomarkers studied are derived not only from the host response to inflammation or bronchial infection, but also from certain characteristics of the infection, such as bacterial load and pulmonary dysbiosis, which have also been associated with severity and response to treatment.96,97
Finally, although the inflammatory response in these patients manifests predominantly in the lung, various blood-based biomarkers that are easily measured in clinical practice, such as CRP or TNF-α, also have a prognostic value.98
Biomarkers in idiopathic pulmonary fibrosis and other interstitial lung diseasesMost research in recent years into new biomarkers in these diseases has focused on idiopathic pulmonary fibrosis and other interstitial lung diseases.99–103 The only biomarkers currently recommended in clinical practice are lung function tests (LFT), radiological findings in chest high resolution computed tomography (HRCT), or histological analysis.104,105 The greatest challenge for the future lies in identifying diagnostic and prognostic biological biomarkers for these diseases, since their course is highly variable and arriving at a firm diagnosis can be difficult without resorting to invasive tests.106 The emergence of new therapies for IPF107 raises the need for new biomarkers that can help evaluate the response to these treatments.108,109
Four areas of research into the pathogenesis of pulmonary fibrosis are currently being explored: epithelial damage/dysfunction; extracellular matrix expression; regulation of the immune system; and genetics. Table 3 shows the most important biomarkers currently being investigated in the field of interstitial lung diseases. None of these promising biomarkers has yet shown significant diagnostic value, as they are not capable of differentiating between different interstitial lung diseases and their prognostic value is similar to that of LFTs. However, future studies could well reveal their true value in the diagnosis of interstitial lung diseases.
Main serological biomarkers in IPF and other ILDs.
| Biomarkers of lung fibrosis | |||
|---|---|---|---|
| Epithelial damage | Extracellular matrix | Immune system | Genetics |
| KL6, SP-A, SP-D, CC16, YKL40 | MMP1, MMP7, LOXL2 | CCL18, IL-6, Osteopontin | MUC5B polymorphisms, Telomere abnormalities |
KL6: Krebs von den Lungen-6; SP-A: surfactant protein A; SP-D: surfactant protein D), CL16: Clara cell protein 16; YKL-40: chitinase-3-Like Protein 1; MMP-1: matrix metalloproteinase 1; MMP-7: matrix metalloproteinase 7; LOXLX2: lysyl oxidase-like 2; CCL18: chemokine (C-C motif) ligand 18; IL-6: interleukin 6; MUC5B: mucin 5B.
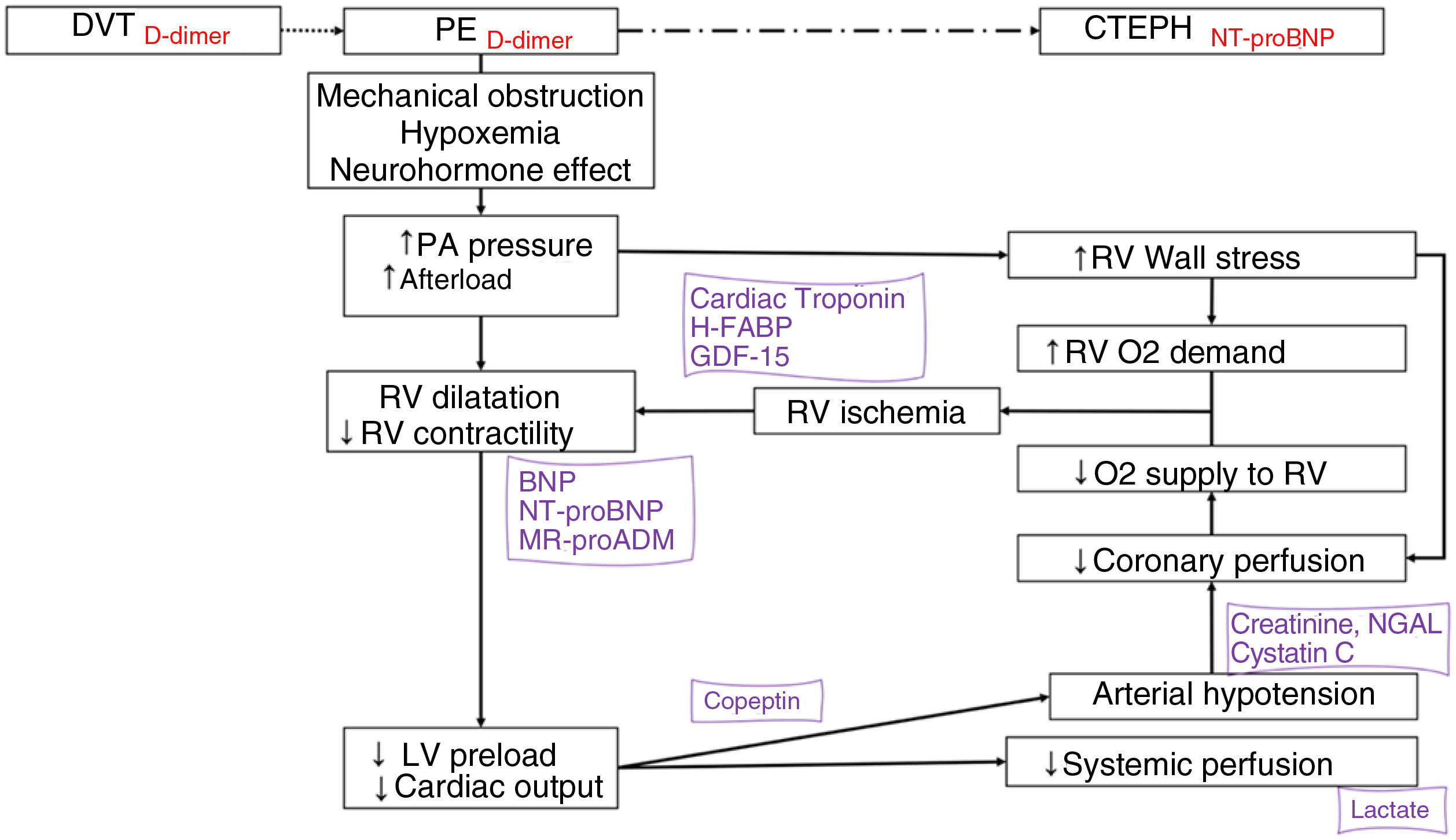
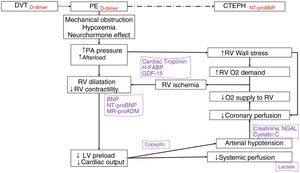
Blood-based biomarkers optimize the diagnosis and treatment of pulmonary embolism (PE) (Fig. 2).
Biomarkers in the pathophysiology of acute pulmonary embolism. The biomarkers are positioned according to the pathophysiological mechanism they express and their diagnostic (in red) or prognosis (in purple) value.
BNP: B-type natriuretic peptide; CTEPH: chronic thromboembolic pulmonary hypertension; DVT: deep vein thrombosis; GDF-15: growth differentiation factor 15; H-FABP: heart-type fatty acid-binding protein; LMR: lymphocyte-monocyte ratio; LV: left ventricle; MR-proADM: mid-regional proadrenomedullin; NGAL: neutrophil gelatinase-associated lipocalin; NLR: neutrophil-lymphocyte ratio; NT-proBNP: amino-terminal fragment of proBNP; O2: oxygen; PA: pulmonary artery; PE: pulmonary embolism; PLR: platelet-lymphocyte ratio; pO2: partial pressure of oxygen; RV: right ventricle.
d-Dimer has a high negative predictive value for the diagnosis of PE, and can rule out PE in patients with low or intermediate clinical probability of PE, or those classified as PE-unlikely.110–114 Furthermore, elevated levels of D-dimer during follow-up are associated with a higher risk of thrombotic recurrence after stopping anticoagulation.115–117 D-dimer has also been useful in excluding PE in patients with COVID-19 pneumonia, although in these cases D-dimer cut-off points differ from those used in routine clinical practice in patients without SARS-CoV-2.118
Various blood-based biomarkers have prognostic value and can be used to stratify risk when combined with clinical and imaging parameters.110,119 The harmful effects of PE on the right ventricle (RV) determine prognosis during the acute phase. The most important markers of myocardial damage are cardiac troponins and heart-type fatty acid-binding protein (H-FABP).120–123 The main blood-based biomarkers of RV dysfunction are B-type natriuretic peptide (BNP) and its amino-terminal fragment (nT-proBNP). These cardiac biomarkers are particularly useful because they can rule out an unfavorable early course.124,125 The addition of other prognostic biomarkers, such as copeptin,126 lactate,127 serum creatinine,128 plasma sodium,129 cystatin C, and neutrophil gelatinase-associated lipocalin,130 could help determine the prognosis in patients with acute PE. Some routine analytical parameters have been associated with an increased risk of occult malignancy at the time of PE diagnosis, including anemia, high platelet and leukocyte counts, and d-dimer levels of more than 4000ng/mL.131–134
Finally, other blood-based biomarkers under investigation could be useful in PE: certain inflammatory markers (IL-6),135 neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio,136 and lymphocyte-to-monocyte ratio,137 growth differentiation factor 15 (GDF-15),138 mid-region proadrenomedullin,139 certain circulating microRNAs,140–142 and the microbiome.143
Biomarkers in pulmonary hypertensionIn pulmonary arterial hypertension (PAH), prognosis is determined by the pathophysiological interaction between the rate of progression of obstructive changes in the pulmonary microcirculation and the adaptive response of the right ventricle (RV). The pathophysiological mechanisms of PAH include vasoconstriction, smooth muscle proliferation, inflammation, endothelial apoptosis, apoptosis-resistant endothelial proliferation, fibrosis, in situ thrombosis, and finally, plexiform lesions, which are a proliferation of what appear to be monoclonal endothelial cells144,145
A large number of PAH biomarkers have been identified, including markers of myocardial dysfunction and injury, inflammation, vascular dysfunction and proliferation, coagulation and platelet activity, hypoxia, and tissue damage,146–151 all of which can be useful in establishing a prognosis (Table 4).
Usefulness of some biomarkers in the diagnosis, treatment and follow-up of patients with pulmonary hypertension.
| 1. | Identify patient population at risk of pulmonary arterial hypertension (PE, systemic sclerosis): NT-proBNP, UA, PIM-147. |
| 2. | Disease progression and response to treatment: BNP, NT-proBNP, ET-1, Ang-2, ADM, PaCO2. |
| 3. | Identify patients with right heart failure: BNP, NT-proBNP, TnI, OPN. |
| 4. | PAH prognosis: BNP, NT-proBNP, TnT, IL-6, IL-8, IL-10, IL-12p70, PCR, OPN, ADMA, vWF, PaCO2, UA, kidney function, Na, copeptin, bilirubin. |
ADM: adrenomedullin; ADMA: asymmetric dimethylarginine; Ang: angiopoietin; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; BUN: Blood Urea Nitrogen; CRP: C-reactive protein; CysC: cystatin C; ET-1; endothelin 1; GDF-15: growth differentiation factor 15; H-FABP: heart-type fatty binding protein; IL: interleukin; miRNA: microRNA; MPV: mean platelet volume; Na: sodium; NT-proBNP: N-terminal propeptide brain natriuretic; OPN: osteopontin; PIM-1: provirus integration site for Moloney murine leukemia virus; PLC: platelet count; TnI: troponin I; TnT: troponin T; UA: uric acid; VSMCs: vascular smooth muscle cells; vWF: von Willebrand factor.
Lung cancer (LC) is the most common cause of death from cancer.152 The biomarkers identified so far range from readily available parameters, such as serum albumin or platelet count,153,154 to others that are more complex to measure, such as genetic mutations or biomarkers associated with the airway microbiome.155–157
In non-small cell LC patients with specific genetic lesions, appropriately targeted therapy improves treatment outcomes compared with standard chemotherapy. It is important to determine target molecular alterations in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), proto-oncogene 1 (ROS 1), B-Raf proto-oncogene (BRAF), neurotrophic tyrosine receptor kinase (NTRK) and programmed cell death-1 (PD-1) and/or programmed cell death ligand-1 (PD-L1) immune checkpoints, as well as tumor mutational burden.158,159 Biomarkers guiding treatment in advanced LC, which have increased in recent years, now include both approved and investigational drugs. It is important to bear in mind that next generation sequencing (NGS) data can show changes and reveal new therapeutic and prognostic biomarkers. Considerable advances have been made in the use of biomarkers in patient selection protocols for screening programs and in the management of incidental pulmonary nodules. Promising results have been obtained from various biomarkers identified in blood and other fluids.160–168 Several multiple biomarker panels have been developed to help clinicians classify indeterminate pulmonary nodules.165–168 However, these panels must be used in an appropriate clinical setting, and require further validation.
Biomarkers in pleural effusionMeasuring biomarkers in pleural fluid (PF) is a rapid, non-invasive method of determining the etiology of pleural effusion (PE). In patients with heart failure, NT-proBNP169 in PF has high sensitivity for determining the cause of the disease, particularly in patients that do not satisfy any Light's criteria. pH and glucose are the most effective biomarkers in guiding decision-making in the management of parapneumonic pleural effusion (PNPE).170 Although many biomarkers of tuberculous pleural effusion (TPE) have been studied, >35IU/L adenosine deaminase (ADA)171 still has the highest diagnostic sensitivity. Many biomarkers of malignant pleural effusion (MPE) have also been studied, although a malignant etiology of PE must still be confirmed by cytohistology. Some of these biomarkers can classify PE according to its etiology; among them, calprotectin172 is the most effective in distinguishing malignant from benign PE. Other validated plasma tumor markers, such as CEA or CA15.3, can indicate the origin of MPE. New molecular biomarkers that can be used to define therapeutic targets and individualize the treatment of LC, such as EGFR, PDL1, ROS1 or ALK, deserve special mention.173 These markers can be measured effectively not only in tumor tissue but also in PE, thus avoiding invasive sampling techniques.
Biomarkers in sleep apneaObstructive sleep apnea (OSA) is a respiratory disorder characterized by total or partial occlusion of the airway during sleep. OSA causes sleep fragmentation, changes in intrathoracic pressure, and episodes of hypoxia-reoxygenation. These events occur repeatedly during sleep, and trigger intermediate mechanisms related to the pathophysiological consequences of OSA, such as sympathetic activation, endothelial dysfunction, hypercoagulability, oxidative stress, inflammation, and metabolic dysregulation. Estimates suggest that between 35% and 40% of variance in the apnea hypopnea index may be explained by familial factors.174 Some experts have suggested that genetic variants in craniofacial structure, body fat distribution, and neural control of upper airway muscles may contribute to the manifestation of different OSA phenotypes.
Numerous studies have explored the usefulness of different diagnostic and prognostic biomarkers for OSA. In adults, the combined analysis of glycosylated hemoglobin (HbA1c), CRP, and erythropoietin (EPO) are useful for OSA screening.175 Furthermore, IL-6 and IL-10 detected in blood have been shown to be robust biomarkers for OSA diagnosis,176 and microRNAs have recently emerged as potential biomarkers for diagnosis177,178 and response to CPAP treatment in patients with resistant hypertension and OSA.179 In children, the combination of kallikrein-1, uromodulin, urocortin-3, and orosomucoid-1 in urine samples has shown excellent diagnostic accuracy,180 and changes in urinary neurotransmitters are good biomarkers for OSA.181
Biomarkers related to the pathophysiological processes of OSA have been identified, mainly associated with sympathetic activation (catecholamines),181 endothelial dysfunction (nitric oxide and adhesion molecules such as vascular cell adhesion and intercellular adhesion proteins182,183), hypercoagulability,184,185 oxidative stress (ROS, isoprostane,186 malondialdehyde),187 inflammation188 (HIF-1α, NF-κß, IL-6 and TNF-α), and metabolic dysregulation. Recent evidence has shown the association between OSA and elevated circulating levels of VCAM-1, which would contribute to tumorigenesis through integrin-based adhesion, and could therefore increase cancer prevalence, incidence, and mortality in individuals with OSA.189 Soluble PD-L1 has also been suggested as a potential biomarker of aggressiveness and metastasis in patients with cutaneous melanoma and OSA.190
Biomarkers in systemic diseases with pulmonary involvementDiffuse interstitial lung disease (ILD) is a leading cause of morbidity and mortality in patients with systemic autoimmune diseases (SAD), and is a particularly common manifestation in rheumatoid arthritis (RA), systemic sclerosis (SSc), and myopathy.191
Serum autoantibodies are currently the only biomarkers available in clinical practice for the diagnosis and classification of SADs.192 Anti-Scl-70 (also known as anti-topoisomerase I), anti-U3-RNP and anti-Th/To antibodies can identify patients at risk of developing SSc-associated ILD.193
In RA, older age, male gender, a history of smoking, and seropositivity for rheumatoid factor (RF) or cyclic citrullinated peptide antibody (CCPA) are risk factors for ILD. Other antibodies against carbamylated proteins and antibodies against peptidyl arginine deaminases (anti-PAD) are also linked to RA.194
Silicosis has also been associated with a higher incidence of systemic autoimmune rheumatic diseases.195
Several antibodies that increase the risk of developing ILD, such as anti-aminoacyl-tRNA synthetase (anti-synthetases) and anti-CADM-140 (MDA5/IFIH1) can be detected in patients with myopathy. ILD is sub-acute in anti-synthetase positive patients, and is particularly aggressive in the case of MDA5 myopathy.196
Progress has been made in recent years in the search for biomarkers other than autoantibodies, such as proteins secreted by alveolar epithelial cells, inflammatory cytokines, and chemokines. IL-6, IL-8, IL-10, CCL2, CXCL10, CX3CL1, fibroblast growth factor 2 (FGF-2), and vascular endothelial growth factor, KL-6, and SP-D have been associated with the presence or progression of ILD in patients with various types of SAD197 (Table 5).
Possible serum biomarkers related to progression in ILD associated with systemic diseases.
| Systemic disease | Biomarkers |
|---|---|
| Systemic sclerosis | - Anti-topoisomerase-1 Ab (Scl-70)- Anti-U11/U12 RNP Ab- Antinuclear antibody Ab staining pattern (indicates anti-Th/To, U3 RNP)- C-reactive protein- IL-6 and IL-10- CCL2 (MCP-1), CXCL4, CCL18- KL-6- SP-D |
| Rheumatoid arthritis | - Rheumatoid factor- ACPA, anti-PAD and anti-carbamylated proteins- HLA-DRB1- MMP-7- KL6- PARC- SP-D- Interferon-γ inducible protein 10 (IP-10 CXCL10) |
| Dermato/polymyositis | - Anti-aminoacyl-tRNA synthetase (anti-synthetases)- Anti-CADM-140 (MDA5/IFIH1).- Ferritin- CRP- KL6 |
| Sjogren's syndrome | -Anti-Ro 52/SSA antibodies-KL6-Angiopoietin-2 protein (Angptl2) |
| Systemic lupus erythematosus | -Extractable nuclear antigens (ENA) |
ACPA: anti-citrulline antibodies; CADM: clinically amyopathic dermatomyositis; CCL: chemokine ligand; CRP: C-reactive protein; CXCL4: chemokine ligand 4; IFIH1: interferon induced with helicase C domain 1; IL-6: interleukin 6; IL-10: interleukin 10; KL6: Krebs von den Lungen-6; MCP1: monocyte chemoattractant protein 1; MDA5: melanoma differentiation-associated protein 5; MMP-7: matrix metalloproteinase 7; PAD: peptidyl arginine deaminase; PARC: pulmonary and activation-regulated chemokine; RNA: ribonucleic acid; SP-D: surfactant protein D.
The practice of precision medicine, in which treatment is tailored for each patient, has been made possible by the discovery of markers, particularly biological markers. Ideally, these markers should be simple and inexpensive to measure in clinical practice, easy to interpret, sensitive and specific for a certain disease (in this case, a respiratory disease), and should be diagnostic, prognostic and/or predict response to treatment. Although research has been able to link some lung diseases with more biomarkers than others, considerable interest in this field in recent years suggests that in the not too distant future researchers will identify biological biomarkers that will help us find homogeneity in the predominantly heterogeneous field of respiratory diseases.
FundingThis manuscript has not received any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.