Tuberculosis (TB) represents a diagnostic and therapeutic challenge for solid organ transplant recipients, particularly after lung transplant (LT). Our aim was to determine the impact of TB in LT patients in Spain, considering prevalence, clinical presentation, prevention and therapeutic management. In addition, differences in outcome between rifampicin (RIF) versus non-RIF containing regimens were analyzed.
MethodsMulticenter, observational retrospective study, including all cases of TB diagnosed in recipients after LT, in five pulmonary transplant units in Spain, between January 1990 and December 2017.
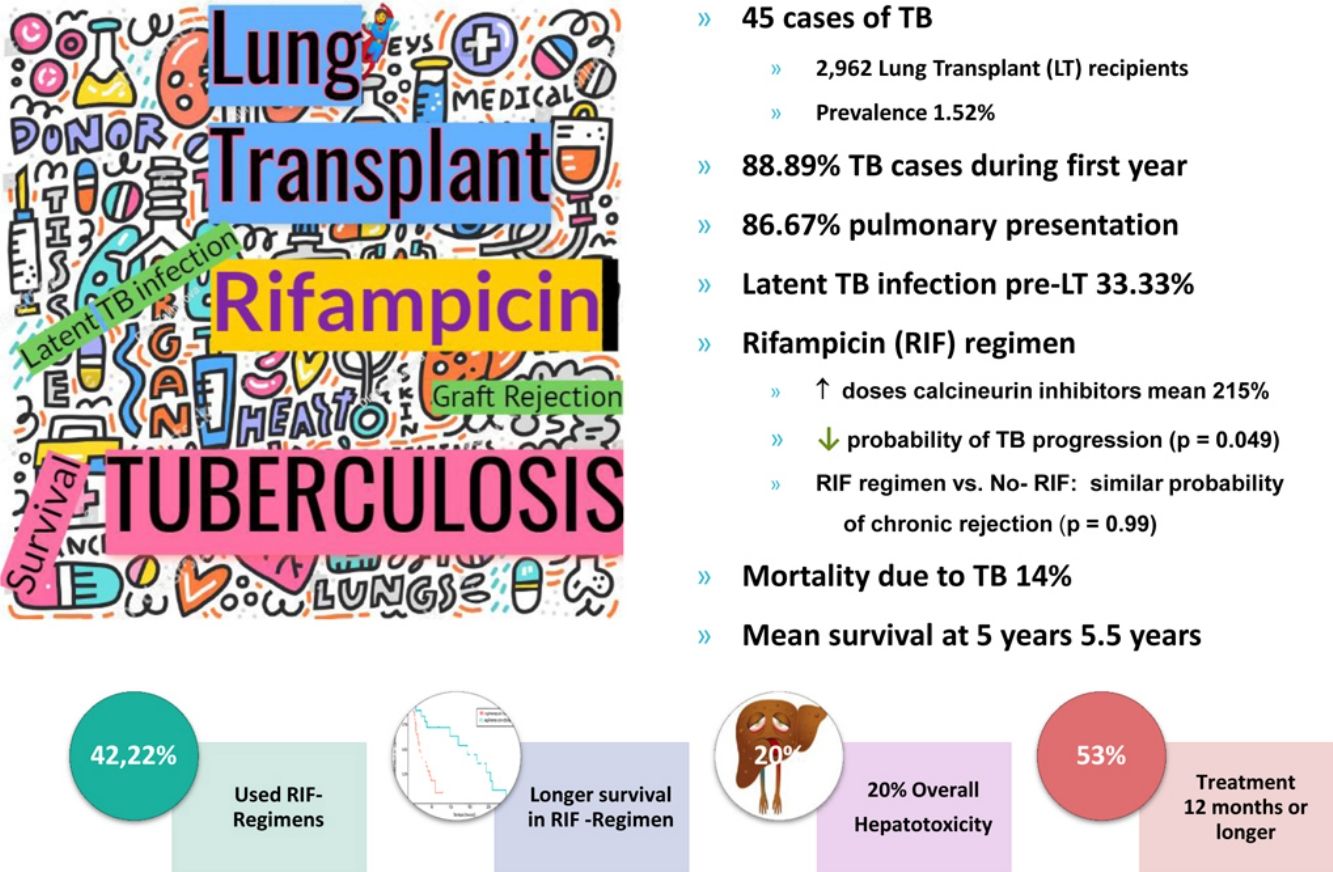
ResultsAmong 2962 LT recipients, 45 cases of TB were diagnosed, resulting in a prevalence of 1.52%. Most of them (88.89%) were diagnosed during the first year posttransplantation, 86.67% with pulmonary presentation. Screening for latent TB infection (LTBI) was done in 36 of the 45 patients and LTBI was detected pretransplant in 12 (33.33%). Less than half of the patients with disease (42.22%) received rifampicin (RIF). Lower probability of TB worsening was found in RIF-containing regimens (p=0.049), as well as longer survival (p=0.001). RIF use was not associated with an increased risk in rejection (p=0.99), but doses of calcineurin inhibitors (CNI) had to be raised an average of 215%.
ConclusionsRisk of TB after LT was lower in our series than previously reported. TB should be searched during the first year posttransplant in patients with TB risk factors. Pulmonary presentation was predominant. More sensitive algorithms for detecting LTBI before LT are crucial. It is reasonable to use RIF-containing regimens over non-RIF regimens based on the tendency toward better outcome in our series. RIF regimen requires close monitoring of CNI trough level for 2–3 weeks, until stability is achieved.
La tuberculosis (TB) representa un reto diagnóstico y terapéutico para los receptores de trasplantes de órgano sólido, en particular tras un trasplante de pulmón (TP). Nuestro objetivo fue determinar el impacto de la TB en los pacientes con TP en España, tomando en consideración su prevalencia, presentación clínica, prevención y manejo terapéutico. Además, se analizaron las diferencias en los resultados finales entre los tratamientos que incluían rifampicina (RIF) frente a aquellos que no la incluían.
MétodosEstudio multicéntrico, observacional y retrospectivo que incluía todos los casos de TB diagnosticados en pacientes receptores de TP, en 5 unidades de trasplante pulmonar en España, entre enero de 1990 y diciembre de 2017.
ResultadosEntre los 2.962 pacientes receptores de TP, se diagnosticaron 45 casos de TB, siendo esta una prevalencia del 1,52%. La mayoría (el 88,89%) se diagnosticaron durante el primer año postrasplante; el 86,67% de ellos fue con presentación pulmonar. Se realizó cribado en busca de infección tuberculosa latente (ITBL) en 36 de los 45 pacientes y se detectó ITBL pretrasplante en 12 de ellos (33,33%). Menos de la mitad de los pacientes con la enfermedad (42,22%) recibieron tratamiento con RIF. Se halló una menor probabilidad de empeoramiento de la TB en los tratamientos que incluían RIF (p=0,049), así como mayor supervivencia (p=0,001). El uso de RIF no se asoció a un aumento en el riesgo de rechazo (p=0,99), pero fue necesario aumentar en una media del 215% las dosis de inhibidores de calcineurina (ICN).
ConclusionesEl riesgo de TB tras un TP fue menor en nuestra serie que lo referido previamente. Debería investigarse la TB durante el primer año postrasplante en aquellos pacientes con factores de riesgo para TB. La presentación pulmonar fue la predominante. Es crucial elaborar algoritmos con mayor sensibilidad para detectar ITBL antes del TP. Es razonable utilizar tratamientos que incluyan RIF frente a aquellos que no la incluyen basándonos en la tendencia a un resultado final más favorable en nuestra serie de casos. Los tratamientos con RIF requieren un seguimiento minucioso de los niveles de ICN durante 2-3 semanas hasta que se alcance una situación estable.
Tuberculosis (TB) represents a diagnostic and therapeutic challenge for solid organ transplant (SOT) recipients, particularly for lung transplants (LT). The prevalence of TB worldwide in SOT is variable, ranging from less than 1% to 6.4%.1–6 In a classic Spanish study conducted on SOT, an incidence of 0.48% was reported. It was particularly high in patients with LTs, with 5.6 times higher risk of developing TB than recipients of other organs and 73.3 times higher than that of the general population.4 European centers have reported a TB prevalence of up to 3.5% in SOT, although more recent series show lower rates, ranging between 0.45% and 0.9%.6,7
Diagnosis of TB in SOT recipients presents challenges that can lead to treatment delays. Classically, these include atypical clinical presentation, with extrapulmonary forms8 and negative sputum smear results despite active disease.4,9,10 In addition, there is an increased likelihood of negative tuberculin skin tests (TSTs) and/or interferon-gamma release assays (IGRAs), tools approved for latent infection (LTBI), also used to help in the diagnosis of active disease.3
TB posttransplant usually develops from a LTBI in the recipient that often goes undetected or is inaccurately treated, but can also develop from a new TB infection or a donor derived infection (DDI) (i.e. USA 4%).2,4,5,11
An emerging issue in developed countries is TB derived from donors coming from endemic TB areas. In Spain, annual TB incidence in donors is two to three times higher than that of general population (30.3 cases/100,000 donors per year) and, currently, 15% of organ donors in Spain are immigrants. In addition, primary resistance to isoniazid (INH) is 3.4% in natives, increasing to 10.2% in immigrants, and primary MDR-TB is 0.1% and 2.2%, respectively.12
Recommendations for the treatment of active TB in transplant recipients are based largely on randomized trials in immunocompetent hosts. Data regarding safety and efficacy of TB therapy in SOT recipients comes from retrospective studies, case reports and case series. The American guidelines (AST) strongly prefer a rifamycin-containing regimen (rifampicin or rifabutin) in any TB scenario (due to its potent sterilizing and bactericidal activity, and prevention of resistance).3 In contrast, the Tuberculosis Network European Trials group (TBNET) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID), suggest a non-rifamycin regimen in cases of localized non-severe TB when there is no suspicion or evidence of INH resistance.2,9 Thus, there is a debate among lung transplant teams regarding which therapeutic approach is better in non-severe TB cases.10 One of the main reasons for this disparity is that rifamycins are potent inducers of cytochrome CYP3A and reduce serum levels of calcineurin inhibitors (CNI) with an increased risk of acute or chronic graft rejection. Moreover, non-rifamycin regimens are less effective bacteriologically, but may prevent the risk of implant loss. They usually include fluoroquinolones or linezolid and their main drawbacks are longer treatment duration and more side effects.2,13,14 In addition, recurrence and high TB resistance rates have been related to rifamycin-sparing regimens.2,9 Therefore, there are no uniform treatment recommendations and teams treat TB according to their own experience.
The main purpose of this multicenter study was to determine TB impact in LT patients in our country. This research included analysis of, prevalence, demographic data and clinical presentation of patients, predictive diagnostic role of LTBI and influence of RIF use on TB outcome, development of chronic graft rejection and survival post LT.
MethodsStudy designThis retrospective non-interventional study was performed in 5 of the 7 Spanish LT Centers, accounting for over 75% of all LT performed in our country. The two centers that did not participate declined the invitation to participate due to overtasks.
Case identification and data collectionThe clinical histories of all LT recipients between January 1st, 1990 and December 31st, 2017, were screened for microbiologically confirmed TB. Patients with TB were identified by cross-reference of the results extracted from the LT Units’ databases and the Microbiology and Histopathology Departments. Disease diagnosis was established when Mycobacterium tuberculosis was cultured or identified by any molecular test from any clinical sample, following standard methods.5
Data collected for analysis from TB patients after LT included donor type (optimal or suboptimal according to standard criteria), demographic features, underlying lung disease, screening for LTBI, type of procedure (uni- or bilateral), immunosuppressive regimen post LT, graft function pre and post TB, anti-TB drugs received, adverse effects and survival with the different treatment regimens.
Pretransplant recipient screening LTBI protocols included a history of potential prior TB exposure or LTBI treatment plus a positive result in either a TST or IGRA. LTBI was considered positive when TST was ≥5mm and/or a positive IGRA result was obtained.4–6 Donor medical history was investigated for the presence of risk factors (alcoholism, homelessness and recent incarceration), including having been born in a high TB endemic area. In addition, objective data such as prior LTBI history or TB confirmed disease and results obtained during donation assessment were evaluated-abnormal chest X-rays (old granulomatous disease, apical scarring) and bronchoalveolar lavage microbiology results.
Regarding treatment of active TB, anti-TB drugs used during the intensive phase were considered for statistical analysis. Treatment duration included both intensive and maintenance phases. The only adverse effect searched for and recorded was hepatotoxicity, defined as elevation of alanine or aspartate transaminase of 3 times the upper limit of normal range. The management of immunosuppressive therapy during TB treatment was at the discretion of the attending physicians. Acute and chronic lung allograft dysfunctions (CLAD) were diagnosed according to ISHLT criteria.15
Outcome after treatment of TB was measured as cured (negative microbiological cultures and no symptoms after a minimum of 6 months of treatment), progression (worsening diseases) or death. Survival time after TB was expressed in months after TB-drug initiation.
Statistical analysisData analysis was done using the R system for statistical computing (version 3.4.3). A descriptive statistical analysis was performed, including central tendency and dispersion and absolute and relative frequencies.
Logistic penalized regressions were carried out to assess the effect of the use of RIF in probability of TB progression and probability of developing chronic rejection.16 In the survival study, a penalized Cox proportional hazards model including use of RIF, type of transplant performed, sex and age as time-varying covariates was done.17 Penalized models allow estimation of coefficients in small datasets. The proportionality assumption of Cox models was examined by visualizing Shoenfeld residuals for each predictor and by performing tests of nonzero slope. Results were expressed by Kaplan–Meier survival curves. Statistically significant results were considered with p<0.05 (95% confidence interval [CI]).
The study was approved by the Ethics Committee of La Fe Hospital, Spain.
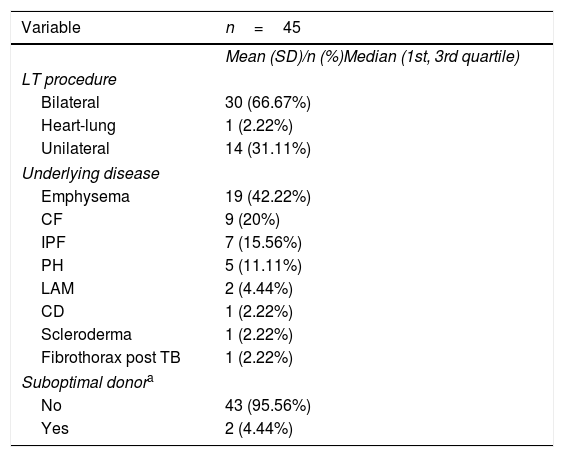
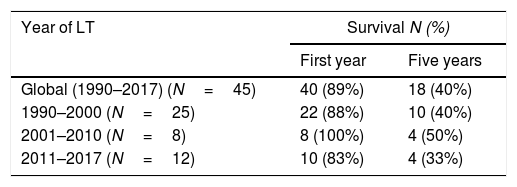
ResultsAmong the 2,962 LT recipients, 45 posttransplant TB cases (1.52%) were diagnosed. Mean age of patients with TB was 45.2 (median 48; Q1=38; Q3=57). Almost half of them were female (46.67%). Underlying disease and type of LT in each patient with TB are shown in Table 1. The incidence of TB in the first decade of the study accounted for 55.5% of the TB cases diagnosed. The median time to TB diagnosis posttransplant was 462.14 days (16.47 months), with 88.89% of patients diagnosed during the first year post-LT (Table 2).
Characteristics of lung transplant recipients who developed TB after lung transplant: Spanish cohort between January 1990 and December 2017.
| Variable | n=45 |
|---|---|
| Mean (SD)/n (%)Median (1st, 3rd quartile) | |
| LT procedure | |
| Bilateral | 30 (66.67%) |
| Heart-lung | 1 (2.22%) |
| Unilateral | 14 (31.11%) |
| Underlying disease | |
| Emphysema | 19 (42.22%) |
| CF | 9 (20%) |
| IPF | 7 (15.56%) |
| PH | 5 (11.11%) |
| LAM | 2 (4.44%) |
| CD | 1 (2.22%) |
| Scleroderma | 1 (2.22%) |
| Fibrothorax post TB | 1 (2.22%) |
| Suboptimal donora | |
| No | 43 (95.56%) |
| Yes | 2 (4.44%) |
CF: cystic fibrosis; IPF: idiopathic pulmonary fibrosis; PH: pulmonary hypertension; LAM: lymphangioleiomyomatosis; CD: ciliary dyskinesia; TB: tuberculosis.
TB cases per decade according to the year of lung transplant, together with survival of recipients with TB in the various time periods.
| Year of LT | Survival N (%) | |
|---|---|---|
| First year | Five years | |
| Global (1990–2017) (N=45) | 40 (89%) | 18 (40%) |
| 1990–2000 (N=25) | 22 (88%) | 10 (40%) |
| 2001–2010 (N=8) | 8 (100%) | 4 (50%) |
| 2011–2017 (N=12) | 10 (83%) | 4 (33%) |
The TB site was mostly pulmonary (86.67%), followed by 2 cases (4.44%) of mediastinal lymph nodes location, and one case each (2.22%) of liver, pleural, genitourinary and disseminated forms. Extrapulmonary TB diagnosis was challenging and the onset was as fever of unknown origin in all cases.
LTBI was detected pretransplant in 12 patients, but TST had not been performed to all recipients during pretransplant assessment; in fact, in 9 of these LT candidates with posterior TB disease, screening was not performed. Even though LTBI had been detected in 12 of the 36 (33.33%) recipients tested, only 10 received pretransplant treatment for LTBI. Only one TB case was a confirmed donor derived infection. In six cases (13.33%), the diagnosis was made from receptor pulmonary explants, indicating a silent active TB prior to transplant that had not been detected in the pretransplant period (sputum smear and culture negatives).
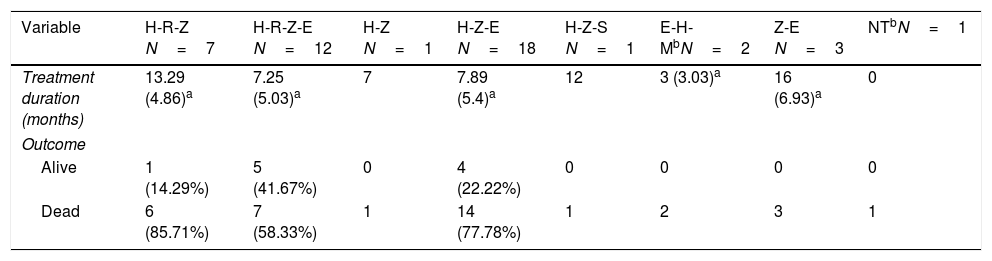
Patients were treated with different combinations of TB drugs (rifampicin [RIF or R] and/or isoniazid [INH or H], pyrazinamide [Z], and ethambutol [E]). The most common TB regimen was triple therapy with HZE (40%); in two cases, moxifloxacin was initially added. Less than half of the patients were treated with RIF, either with triple (15.56%) or quadruple (26.67%) initial combination therapy (HRZE) (Table 3). The median length of TB treatment was 8.64 months. In 53.33% of cases the duration of TB treatment was twelve months or more. Nine patients (20%) developed hepatotoxicity, but only in one patient all TB drugs were discontinued.
Description of the different TB drug combinations used and outcomes (1990–2017).
| Variable | H-R-Z N=7 | H-R-Z-E N=12 | H-Z N=1 | H-Z-E N=18 | H-Z-S N=1 | E-H-MbN=2 | Z-E N=3 | NTbN=1 |
|---|---|---|---|---|---|---|---|---|
| Treatment duration (months) | 13.29 (4.86)a | 7.25 (5.03)a | 7 | 7.89 (5.4)a | 12 | 3 (3.03)a | 16 (6.93)a | 0 |
| Outcome | ||||||||
| Alive | 1 (14.29%) | 5 (41.67%) | 0 | 4 (22.22%) | 0 | 0 | 0 | 0 |
| Dead | 6 (85.71%) | 7 (58.33%) | 1 | 14 (77.78%) | 1 | 2 | 3 | 1 |
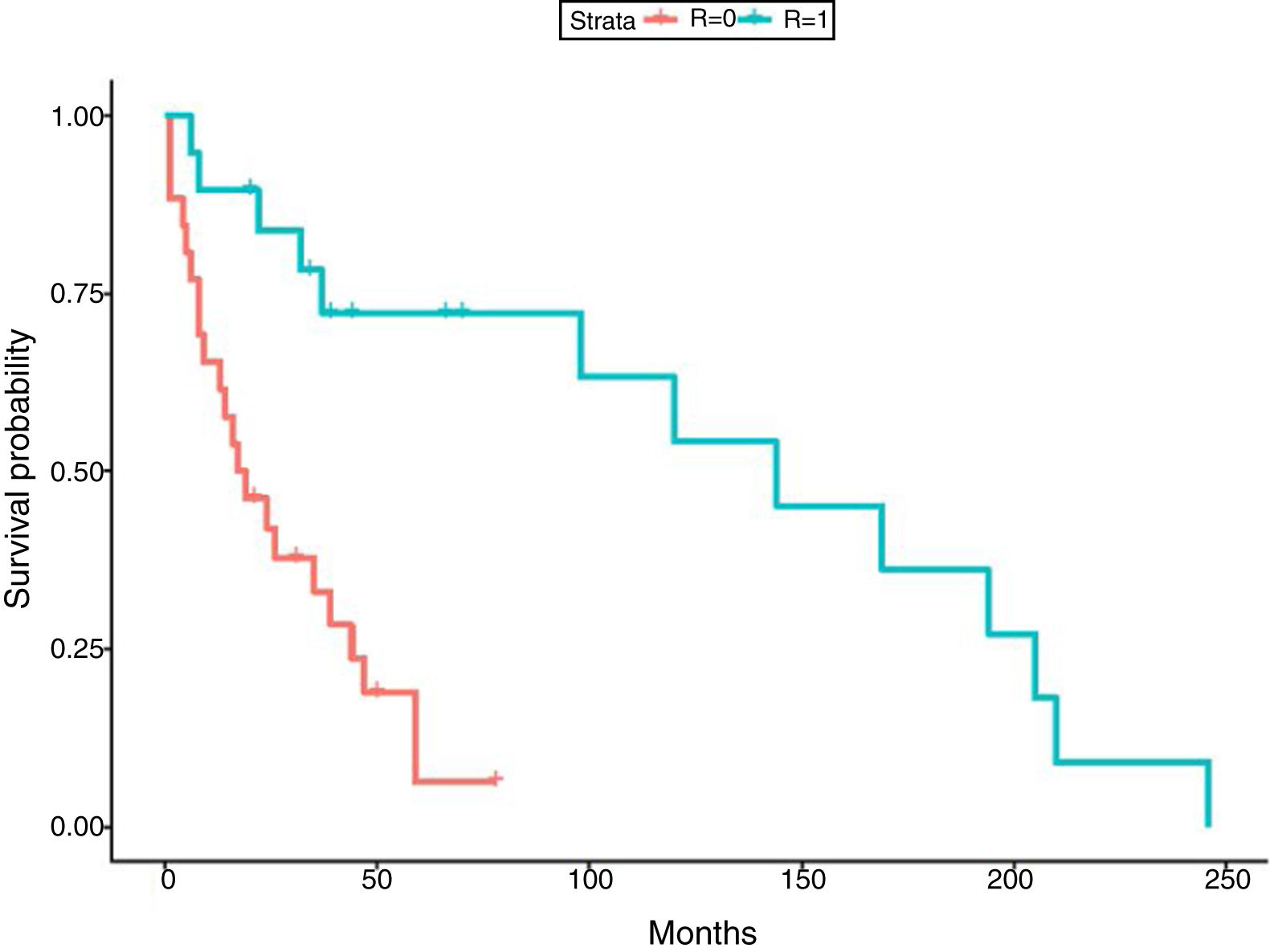
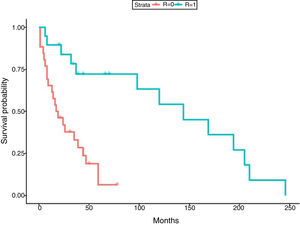
In relation to the treatment chosen, outcomes of TB according to the international definitions were as follows: 33 cured (17 patients with RIF-associated regimen), 11 progressed (only 1 in patient with RIF-regimen) and 1 relapse (no RIF-regimen). All TB-related deaths accounted in non-RIF regimens. The use of RIF was associated with a lower probability of TB progression (odds ratio [OR] 0.19; 95% CI 0.037–0.98; p=0.049) and longer life expectancy. No significant differences were found between the use of RIF and the probability of developing chronic rejection (CLAD) (OR 1; 95% CI 0.3–3.4; p=0.99). Only a documented case of immune reconstitution inflammatory syndrome was reported and no new acute rejection episodes were observed during the RIF regimen. The use of RIF showed a decrease in the risk of death (HR 0.179; p=0.001) (Fig. 1). Other variables, such as type of transplant performed, sex and age did not influence in this result.
The most common immunosuppression combination used (51.11% of cases) was cyclosporine, azathioprine and prednisone, followed by the combination of tacrolimus, mycophenolate mofetil and prednisone, especially in the last decade. An increase in CNI dosages by an average of 215% (range 50% to 333%) in RIF regimen was necessary.
The average survival time from transplant to death was 66.69 months. Survival one year after transplantation was 88.89% (95% CI 0.8–0.99), and survival at 5 years was 40% (95% CI 0.32–0.62) (Table 2).
Cause of death could be identified in 35 of the 45 patients. Mortality attributed to TB occurred in 14.29% of cases. In a high percentage of patients (51.43%) the cause of death was miscellaneous, but not due to progression of TB. As already described in LT, death due to CLAD was 34.29% during follow up.
DiscussionThis review is the largest and most current TB series on LT recipients and adds information to the paucity of data known about TB after LT. The prevalence of TB in our series was lower than the previously reported in two LT Spanish series, 6.41%18 and 2.58%,19 likely due to the significant decrease in TB in Spain in the last 15 years. However, it is still twice the prevalence described in other developed countries.20
Regarding clinical presentation, the most frequent form was pulmonary (86.67%) similar to that observed in the general Spanish population.21 Extrapulmonary presentation was less common than previous reported in SOT.8 In fact, it has been described that up to half of all cases of active TB after transplant are disseminated or occur at extrapulmonary sites. Furthermore, TB may have an atypical presentation in SOT (i.e. pyomyositis, cutaneous ulcers or abscess, tenosynovitis).8
It is important to point out that most of the TB cases occurred in the first year post-LT. Actually, our early debut was significantly higher than that reported in other series (16.4 vs. 56.42 months).20 Early onset could reflect greater incidence of LTBI than that detected during pretransplant assessment, as well as inadequate handling of LTBI in some cases.
An interesting fact is that 20% of the LT candidates with confirmed TB had not been screened for LTBI and 2 of the positive LTBI patients did not receive treatment for LTBI with isoniazid despite having been diagnosed of LTBI. These deviations from the recommended TB screening guidelines occurred in the first decade of the study. In our series, a higher percentage of patients with TB had been screened for LTBI than the one described in a Spanish SOT cohort in which only 45.4% of the recipients had a TST performed. Around 67% of TB cases had negative TST pretransplant, similar to previous reports.1 In fact, it is well known that tests for detecting LTBI (TSTs and IGRAs) have lower sensitivity in patients with advanced diseases because both are dependent on the cellular immune response of the host to the pathogen and the use of corticosteroids or a sarcopenic condition may decrease cellular immunity.22–27
The most frequent form of acquiring TB after transplant is after LTBI in the receptor, and very unusually as a primary infection or DDI. Donor-derived TB transmission has been reported in SOT for less than 5% of all active TB cases and has a significant morbidity and mortality.2,3,12,28,29 However, an increase of donor-transmitted TB is expected, due to changes in donor profile, globalization and new migratory patterns.11,12 Based on the Spanish TB Consensus Document and data from our organ procurement organization (ONT),12,28 the annual incidence of TB in actual donors is approximately twice that observed in the general population. The higher incidence could be explained by the meticulous screening techniques performed in potential donors, detecting silent cases otherwise undetected. In our series, one confirmed DDI was diagnosed and after 10 years of follow up this patient is alive.
Currently, the optimal combination of TB drugs is controversial in transplant recipients. There is no full agreement in non-complicated TB cases regarding the use or not of a rifamycin, due to drug interaction with immunosuppressants. This can increase the risk of graft rejection by drastic reductions in therapeutic levels for long time resulting in chronic lung rejection. This dilemma is reflected in our series, in which 42.22% of the cases included RIF versus non-RIF regimens.
In our experience, the doses of CNI had to be increased, with an average dose of 215% when RIF was used as previously reported in SOT.8 In fact, the current recommendations advice to increase three times the dose of cyclosporine, tacrolimus or mTOR and oral corticosteroid dose should be increased by 50% initially in a rifamycin regimen.2 An excellent option is to replace RIF with rifabutin, which has similar activity against M. tuberculosis, but is a much less potent inducer of cytochrome P3A4 and, therefore, immunosuppressant levels may be easier to maintain.30 If, finally, a RIF-regimen is chosen, it is important to closely monitor CNI trough and m-TOR levels during the initial days of treatment.
Another concern in the management of TB is the side effects of TB drugs. In our series, hepatotoxicity occurred in 20%, but treatment was only stopped in one patient who finally died from TB progression, after trying different drug combinations for six months. Bodro et al.31 described hepatotoxicity in 39% of SOT recipients. However, that series involved all types of solid organ transplants, including liver, which could explain the higher rate of hepatotoxicity detected.
Other issue in TB treatment is the optimal duration, because no clinical trials have analyzed it. Usually, 9–12 months are recommended in the case of regimens with rifampicin, and 12–18 months for those in whom this drug is not used.2,5,32 In our analysis, most TB treatments lasted for 8 months or longer.
Finally, although regimens without RIF have shown correct disease control,8,33,34 in our study RIF-regimen was associated with lower risk of TB progression and better survival. In fact, all deaths by TB occurred in non-RIF regimens. The mean survival time in our study was 66.69 months (5.5 years), which is similar to global survival times shown in the International Registry of Heart and Lung transplantation35 and in the Spanish registry.36 The similar survival highlights the fact that TB is not currently an important cause of mortality if detected early and treated adequately.
Despite being the most extensive and updated series of TB in LT patients, this study presents a series of limitations when interpreting the results, mainly due to the long time of retrospective data collection. Aspects such as improvements in diagnosis, differences in LTBI protocols, surgical techniques, immunosuppression therapy and new TB drugs overtime should be taken into account. However, despite the low scientific evidence from this type of study, the results allow us to suggest that it would be reasonable to use RIF-containing regimens over non-RIF-containing, based on the trend to a better outcome in our series. It should be mandatory to evaluate pretransplant LTBI TST and IGRAs, due to the high percentage of false negatives with current screening protocols. In addition, based in our results, a close monitoring of LT recipients with LTBI pretransplant or from a risk donor is suggested during the first year posttransplant.
Our results highlight the need for additional research in the field of TB in SOT, including improvements in LTBI diagnostic and treatment protocols, specific follow-up recommendations in recipients with TB risk factors and, finally, implementation of a registry of outcomes with different TB treatment managements after LT.
Conflict of interestThe authors declare no conflict of interest.
Dr. Oscar Len for his review and contribution to this article.