Respiratory diseases exhibit diverse patterns in prevalence, clinical presentations, and outcomes between men and women. Historically, certain conditions were more prevalent in men, but trends have shifted, highlighting the need to understand sex disparities in respiratory health. Social, environmental, and healthcare changes have reshaped the landscape of respiratory diseases, complicating diagnosis and treatment. Moreover, the underrepresentation of women in clinical trials has limited our understanding of their specific needs. In this review, we explore the sex differences in the prevalence, clinical characteristics, and presentation of respiratory diseases, emphasizing the importance of tailored approaches to diagnosis and management. By recognizing and addressing these disparities, we can advance toward more equitable and effective respiratory healthcare for all individuals.
Several respiratory diseases occur predominantly in women, while other conditions that were almost exclusively found or more prevalent in men, have increased among women in the last several decades. Some diseases may also affect women differently and present with greater symptoms and/or severity than in men. Sex plays a relevant role in the development, prevalence and manifestations of the disease, but also in response to treatment. Changes in social roles, exposures and approach to illness have modified the nature and outcomes of some diseases. As up until more recently, women have had a smaller presence or have been completely excluded from randomized clinical trials, we have historically lacked data in this population.1 In this review, we aim to evaluate the sex differences in prevalence, clinical presentation and characteristics for some of the most frequent respiratory diseases.
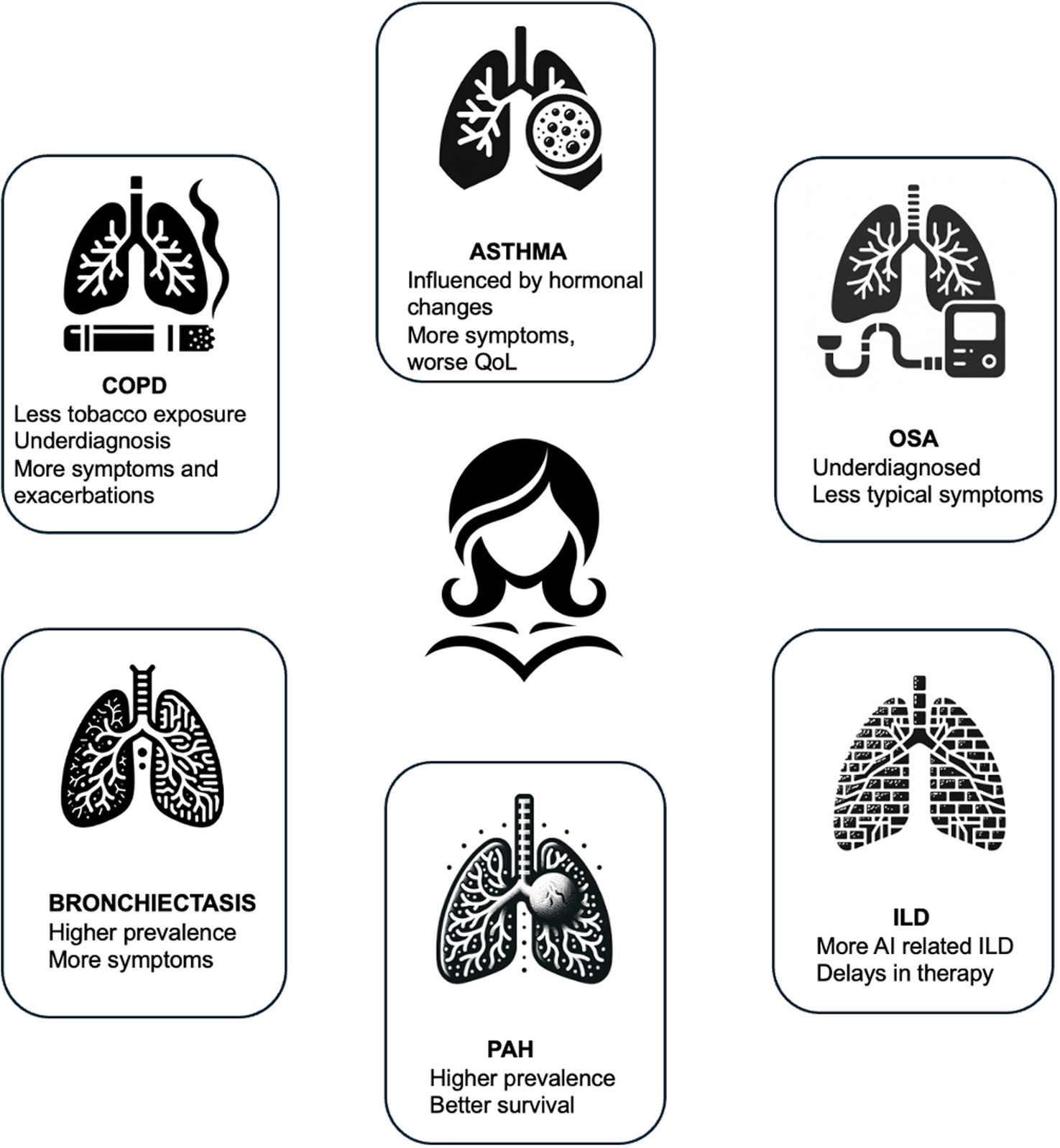
Chronic Obstructive Pulmonary Disease (COPD) in WomenChronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide, affecting women at rates that are becoming comparable to men.2–6 However, women are developing COPD at younger ages and with less tobacco exposure.7,8 They also report more dyspnea and have higher incidence of exacerbations as compared to men with similar airflow limitations.9 In addition, women have not experienced the same improvements in mortality as men in recent years.3–6 Given these inequalities, it is crucial to work toward earlier diagnosis and optimized treatment among female patients.10
COPD is known to be underdiagnosed in women.11 There are likely many contributors including differences in exposures, symptom presentation variance (less coughing and phlegm, but more dyspnea and anxiety/depression), and issues with spirometry-based diagnosis.6,9,11 Women are slightly more likely to have preserved ratio impaired spirometry (PRISm; defined as FEV1<80% predicted and FEV1/FVC≥0.7).12 Despite patients with PRISm having higher rates of development of COPD, and higher all-cause and respiratory mortality, optimal management for these patients remains unknown.12 It is therefore important to continue to study these precursor conditions that provide a window of opportunity to facilitate earlier preventative and therapeutic measures.
When women are ultimately diagnosed with COPD, ensuring appropriate treatment is essential to mitigate their increased risk for exacerbations. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) committee recently updated its guidelines in 2023.13 One major change was a shift in nomenclature from the ABCD group classification to ABE, where group E are patients at increased risk for exacerbations, regardless of symptoms. The first line of treatment for Group E is now long-acting beta-agonist (LABA)+long-acting muscarinic antagonist (LAMA), with consideration for triple therapy (LABA+LAMA+inhaled corticosteroid [ICS]) if absolute blood eosinophils≥300cells/μL. This latter recommendation comes after 2 trials, IMPACT and ETHOS, demonstrated lower rates of COPD exacerbations with triple therapy as compared to LAMA/LABA.14,15 Other promising advances include use of biologic therapies for COPD with type II inflammation. In a recent trial, dupilumab was found to be associated with fewer exacerbations, as well as better quality of life and lung function than placebo.16 This is particularly salient for our female patients who have higher rates of asthma/COPD overlap than males.17
While there remain many challenges to optimizing care for women with COPD, there have been important advances. We have begun to understand COPD as a continuous spectrum of disease which may facilitate earlier detection. In addition, there have been important changes to therapeutic recommendations which have the potential to improve outcomes for patients with COPD, but particularly women.
Asthma in WomenAsthma is a heterogeneous disease of the airways18,19 and gender differences have been observed in prevalence, inflammatory phenotypes and asthma severity. During childhood, asthma prevalence is lower in females, while as adults, women have an increased prevalence and severity of asthma. Cluster analysis revealed differences according to sex with a women predominance in less atopic or in more symptomatic obese patients.20,21 Other studies found a reduced proportion of women in severe eosinophilic asthma associated with nasal polyps.22
Different factors could explain these sex differences. Women are exposed to several hormonal changes with fluctuations of estrogen and progesterone levels during menstrual cycle, pregnancy and menopause. It has been reported that one fifth to one third of women have an increase in asthma symptoms during the pre-menstrual period associated with a decrease in lung function23,24 and an increased in type-2 markers such as sputum eosinophil counts and FeNO (fractional exhaled nitric oxide).25 Progesterone has been shown to inhibit cilia beat frequency when activating its receptor expressed on the surface of airway epithelial cells. Moreover, estrogen receptor (ER)-α activation was associated with an increased bronchial hyperresponsiveness (BHR), IL-33, type-2 cytokines, and airway eosinophilic inflammation. Through ER-β,26,27 estrogen has however been shown to reduce BHR and eosinophilic inflammation.
During pregnancy, changes in asthma symptom control were observed in a small proportion of women.28 Women with more severe asthma prior to pregnancy seem at higher risk of worsening.29 Finally, some authors have proposed a “menopause-associated onset of asthma” phenotype, but this population has a higher prevalence of comorbidities such as obesity, anxiety and depression, and sometimes treatment with hormone replacement therapy (HRT). An increased risk of asthma has been reported in women receiving HRT, in a dose-dependent manner, especially in low BMI patients.30 Hormonal contraceptive use, which prevents the harmful effect of fluctuations in estrogen and progesterone levels, has also been associated with a reduction in asthma incidence and a better symptom control. On the other hand, increased serum testosterone levels have been associated with decreased asthma prevalence in a dose-dependent manner and was associated with increase in lung function.31
Several studies have also highlighted sex specific single nucleotide polymorphisms in genes32,33 involved in immune pathways such as mediated by thymic stromal lymphopoietin (TSLP). Interesting studies also determined that DNA methylation due to environmental exposures and sex hormones are linked to asthma susceptibility.34 Last but not least, women have a higher prevalence of hair product-related allergy and are more likely to have asthma symptoms when exposed to inorganic dusts.35 There could also be differences in physical activity and eating habits between men and women.
In conclusion, data in the literature support the role of sex hormones on asthma incidence and severity. The underlying mechanisms and the different effect of hormones fluctuations and relationship with environmental exposures on asthma risk and symptoms control remains however unclear.
Women and Interstitial Lung DiseasesSex impacts diagnosis, risk factors and management of interstitial lung diseases (ILD) in different ways. Epidemiological studies of idiopathic pulmonary fibrosis (IPF) have consistently shown a predominance of men, with only 20–45% of patients with IPF being female, although this varies across studies.36–41 In contrast, women are more likely to have autoimmune rheumatologic diseases, and therefore autoimmune-related ILD is also more common in women.42,43 One specific ILD diagnosis, lymphangioleiomyomatosis (LAM), a cystic lung disease, occurs almost exclusively in women. Such diagnostic differences across ILD may be due to biological factors, or to environmental factors. Sex hormones are postulated to play a role in sex-based differences noted in animal models of ILD, whereby estrogen seems to impact immune reactivity and inflammatory response leading to a protective effect of on disease severity, while male hormones exacerbate the fibrotic response.44–46 Estrogens are believed to play a pathogenic role in LAM since it is restricted almost exclusively to women, is exacerbated by exogenous estrogen use including oral contraceptives, worsens with the onset of menses,47 and pregnancy.48–50 Genetic studies of familial ILD led to the discovery of mutations in genes involved in telomere homeostasis.51 In vitro, androgens and estradiol both seem to have an effect on telomerase activity.52 Among patients with telomere gene mutations, men are younger than women at ILD diagnosis,53 and their telomere lengths are significantly shorter, suggesting that women are protected from telomere shortening, which is itself a risk factor for ILD.54
In addition, ILD can also be impacted by several sex and sex-related factors (variables that differ between men and women)55,56 such as smoking57 occupation/employment, exposures, and socioeconomic status. Smoking is strongly associated with an increased risk of IPF, particularly with a greater than 20 pack year smoking history.58,59 Occupational exposures to asbestos, silica, and mixed dusts, vapors, gases and fumes are less common in women, due to gendered exposures whereby women are less present in industrial and labor workforces, leading to a predominance of men with pneumoconiosis.60–63
Female sex has been shown in multiple observational studies to be a favorable prognostic factor for disease progression and survival in IPF and other ILD, including autoimmune related ILD.64–66 Sex is a key prognostic marker that has been incorporated into routinely used prognostic scores for ILD, such as the Gender, Age, Physiology (GAP) score.67–69 In a German administrative-data study, women with ILD also had lower hazard of respiratory and all-cause hospitalizations compared to men.70
Although the benefits of ILD treatment does not differ between men and women, management of ILD has been shown to be unequal in women, compared to men. A large American study using medication insurance administrative databases has shown significant disparities in antifibrotic prescriptions for IPF between men and women, whereby men were more likely to receive antifibrotic medications compared to women (30% vs. 22%).71 In an observational study evaluating treatment initiation of patients with ILD across 3 distinct prospective cohorts, women in Canada started ILD-related medications later following diagnosis compared to men, especially in those with a diagnosis of IPF, after adjusting for disease severity.72 This was not seen in the other cohorts. A 236-patient study from France has shown that women with IPF were much less frequently referred for lung transplant compared to men.73
Obstructive Sleep Apnoea (OSA) in WomenSleep apnoea, characterized by reduction in airflow (hypopnea), and cessation of breathing (apnoea) during sleep, is a highly prevalent disease associated with significant increase of morbidity and mortality. Obstructive sleep apnoea (OSA) in particular, where airflow reduction and cessation are caused mainly by an obstruction in the upper airway, is predominantly a disorder in men,74 and for this reason the vast majority of the literature in the field has often neglected the different pathophysiology, clinical manifestations and consequences of such sleep disorder in women. This is clinically relevant as women with undiagnosed severe OSA have a 3.5-fold increase in the incidence of cardiovascular mortality after adjusting for confounders.75 From a pathophysiological standpoint, women, compared to men are characterized by different hormonal fluctuations during their life span.76 In particular, progesterone and estrogen levels tend to rise in adolescence and decline in menopause whilst varying significantly within the menstrual cycle and during pregnancy. Progesterone is a ventilatory drive stimulant and appears to increase the activity of the genioglossus muscle therefore dilating the upper airway.77 Oestrogens also seem to be protective against OSA: animal studies indicate that oestradiol can reduce the oxidative stress brought on by prolonged intermittent hypoxia during OSA.78 Furthermore, there are sex-specific differences related to anatomy: the different body fat distribution and the relatively shorter pharyngeal length compared to men can favor a reduced upper airway collapsibility, better muscle compensation and lower respiratory arousal thresholds.79 These features can explain the relatively shorter duration of obstructive events in women, characterized by more hypopnoea events and fewer apnoea events and less oxygen desaturation and the predominance of REM-related OSA. Regarding clinical manifestations, while male patients with sleep disordered breathing complain of the typical OSA symptoms like snoring, unrefreshing sleep and daytime sleepiness, female patients tend to report symptoms like fatigue, poor energy levels and mood disturbances.80–81 This can at least in part explain why OSA can be under diagnosed and wrongly classified in women with inevitable consequences on treatment and outcomes. Pregnancy deserves to be mentioned as it is a sex-specific physiological event that can predispose to sleep breathing disorders due to hormonal changes, weight gain and changes in ventilatory control and upper airway anatomy.82 When diagnosed, OSA in pregnancy can have an impact on maternal outcomes such as preterm birth.83 OSA has also been associated with gestational diabetes and increased risk of hypertensive disorders of pregnancy.82 OSA tends to be more prevalent in post-menopausal compared to pre-menopausal women.84 This is not only due to age but possibly also to changes in the quantity and distribution of body fat, differences in endogenous sex hormones, such as estrogen and progesterone, and pharyngeal dilating muscle activity. Hormone replacement therapy is supposed to play a role in improving OSA severity, however, interventional randomized controlled studies are scarce and the results of such trials taken together do not allow to prescribe hormonal treatment to treat OSA in menopausal women.85
Bronchiectasis in WomenBronchiectasis is the third most common airway disease following asthma and COPD, with a prevalence estimated at 250–500:100,000, or 0.25–0.5% of the population.86–89 The prevalence of bronchiectasis in various reports is higher in women compared to men, with women comprising 55–70% of individuals with bronchiectasis in Western countries.86,90,91 Surprisingly, women dominance has been reported even in adults with Primary Ciliary Dyskinesia (PCD), a genetic disease of autosomal-recessive inheritance that results in bronchiectasis.92 Women predominance is not universal, however. In reports from India, bronchiectasis was more prevalent in men (56.9% men in the Indian bronchiectasis registry),93,94 and similarly, men dominance is reported in Eastern European countries.95 In children with bronchiectasis, the prevalence of bronchiectasis was reported to be slightly higher in boys than in girls in indigenous populations.96 Other than prevalence difference, women with bronchiectasis have been found to have milder severity, but to be more frequently infected with Pseudomonas aeruginosa (PA), and report a worse quality of life compared to men when adjusted to age and bronchiectasis severity. Women were found more likely than men to receive guideline-recommended bronchiectasis care including aetiologic testing, pulmonary rehabilitation and airway clearance instructions, and long-term antibiotics.95
Sex imbalance in bronchiectasis prevalence may stem from different aetiologies, as well as structural, hormonal, chromosomal, and behavioral influences. In a European bronchiectasis registry (EMBARC) analysis, COPD and smoking were reported more often in men with bronchiectasis, while asthma and connective tissue diseases were more prevalent in women with bronchiectasis.95 The combination of COPD and bronchiectasis has been established as an independent predictor of severity97–100 which may explain the greater severity in men. Differences in chest structure between men and women have been implicated in the elevated prevalence of non-tuberculous mycobacteria pulmonary disease (NTM-pd) among women,101 where middle-lobe predominant bronchiectasis is more common in women102 (Fig. 1). Hormonal differences – mainly the effect of Estrogen – have been implicated, as estrogen was reported to increase PA alginate production and mucoidity.103 In women with cystic fibrosis (CF), pulmonary exacerbations were reported to be more frequent during the follicular phase of the reproductive cycle and to coincide with elevated blood estrogen levels. Furthermore, data from the Irish CF registry showed that oral contraceptive use reduced pulmonary exacerbations.103 Hormonal responses thought to affect sex differences in CF may not be equally influential in bronchiectasis, with a mean age of above 60, when menopause is accompanied by decreases in levels of female sex hormones to levels below those in males. Immune responses may be different in women and men, increasing inflammatory responses in women since the time of puberty.104 Behavioral differences may include differences in recognition of symptoms and turning to medical care for the diagnosis, as well as in adherence to treatment recommendations.95
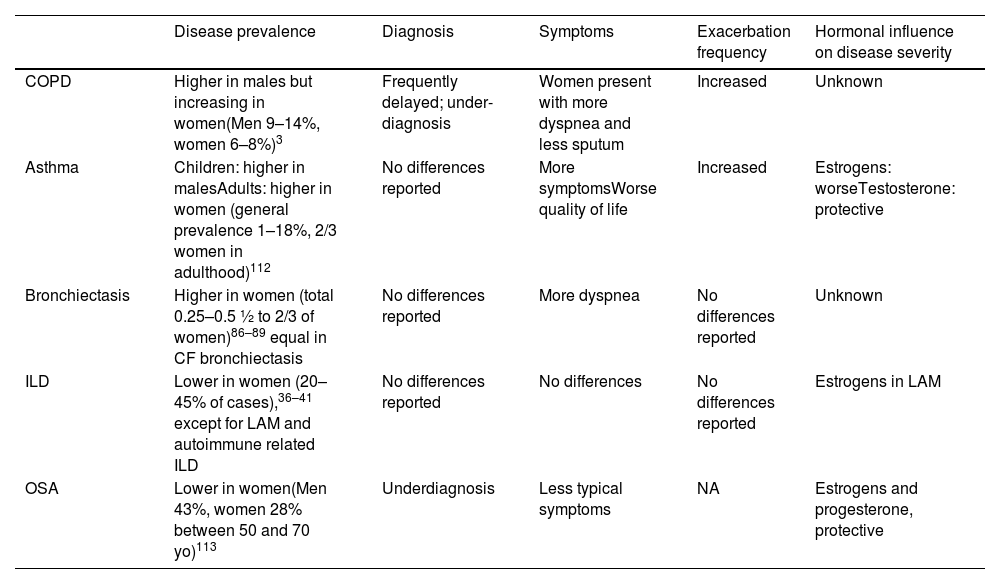
Some women- specific issues may be encountered more frequently in women with bronchiectasis. Women with bronchiectasis are more frequently affected than men by stress urinary incontinence,105–107 which should be actively sought and treated. Young women (and men) with bronchiectasis secondary to PCD (primary ciliary dyskinesia) were reported to have increased rates of subfertility108 and ectopic pregnancies, with contradicting findings between studies.109 Data on pregnancy outcomes in young women with bronchiectasis are scarce. A single-center retrospective study has found a lower rate of live births (0.77±0.3 vs. 0.9±18, p=0.02), and a trend toward increased rate of low birth weight and neonatal malformations among women with bronchiectasis compared to controls.110 The European Respiratory Society/Thoracic Society of Australia and New Zealand (ERS/TSANZ) Task Force Statement on the management of reproduction and pregnancy in women with airways diseases recommends attention to nutritional needs and maintaining airway clearance throughout pregnancy.111Table 1 summarized differences in epidemiology and symptoms between men and women.
Differences in Epidemiology and Symptoms Between Men and Women.
| Disease prevalence | Diagnosis | Symptoms | Exacerbation frequency | Hormonal influence on disease severity | |
|---|---|---|---|---|---|
| COPD | Higher in males but increasing in women(Men 9–14%, women 6–8%)3 | Frequently delayed; under-diagnosis | Women present with more dyspnea and less sputum | Increased | Unknown |
| Asthma | Children: higher in malesAdults: higher in women (general prevalence 1–18%, 2/3 women in adulthood)112 | No differences reported | More symptomsWorse quality of life | Increased | Estrogens: worseTestosterone: protective |
| Bronchiectasis | Higher in women (total 0.25–0.5 ½ to 2/3 of women)86–89 equal in CF bronchiectasis | No differences reported | More dyspnea | No differences reported | Unknown |
| ILD | Lower in women (20–45% of cases),36–41 except for LAM and autoimmune related ILD | No differences reported | No differences | No differences reported | Estrogens in LAM |
| OSA | Lower in women(Men 43%, women 28% between 50 and 70 yo)113 | Underdiagnosis | Less typical symptoms | NA | Estrogens and progesterone, protective |
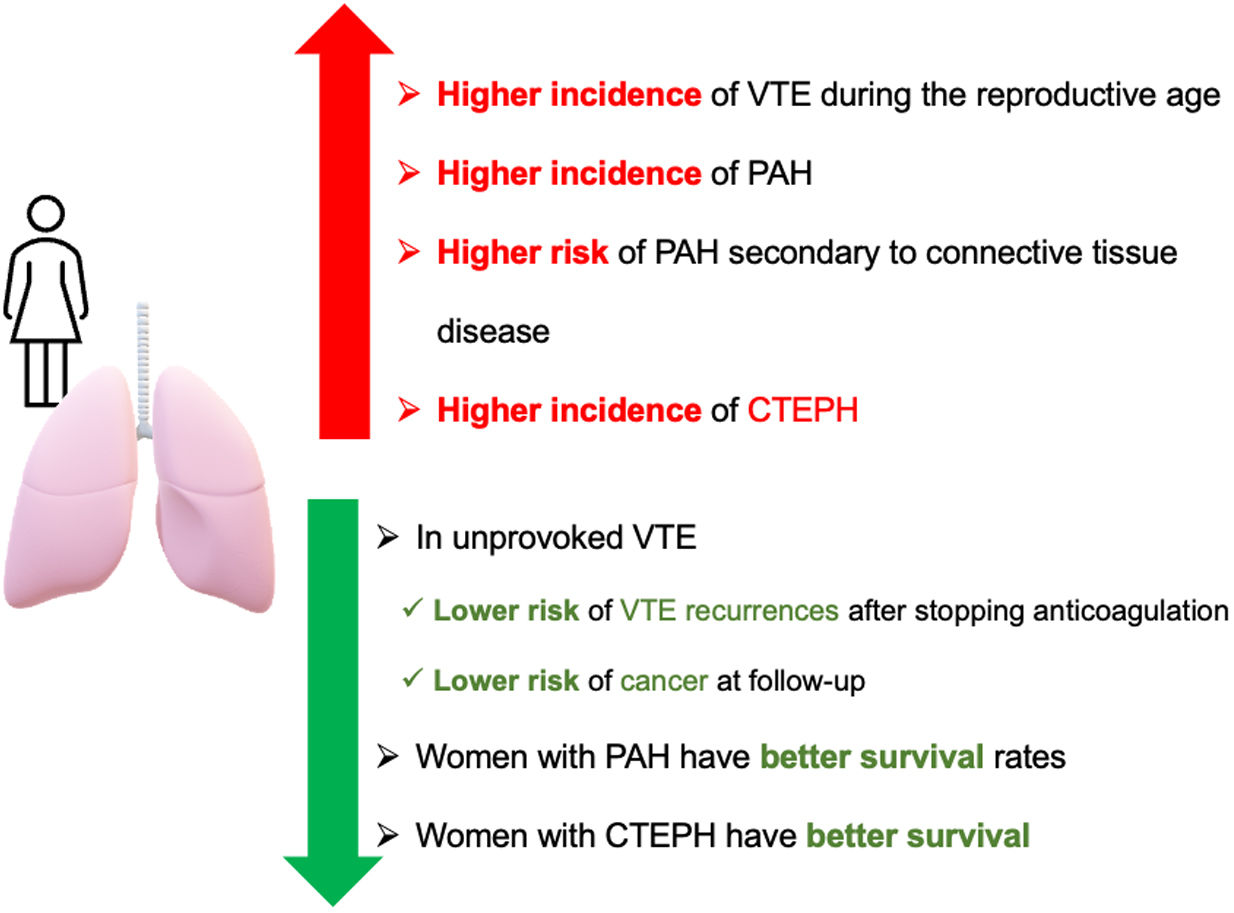
PE is globally the third most frequent acute cardiovascular disease following myocardial infarction and stroke.114,115 In women, the incidence of VTE is higher during the reproductive age, which can be explained by pregnancy and hormonal treatments. However, from the age of 45 onwards, the incidence is higher in men.116
Optimal anticoagulant treatment duration depends on whether the risk factor is persistent, transient, major, or minor.117 For patients with unprovoked VTE (venous thromboembolism), the recommendation is to undergo long-term anticoagulant treatment.114,115,118 In this population, scores have been developed to safely discontinue anticoagulant treatment, where the female sex is considered a protective factor due to its lower incidence of recurrent VTE.119–121
Occult cancer is detected in 5% of patients within 1-year after unprovoked VTE, and the risk for females is lower than for males.122 Furthermore, females showed more heterogeneity in cancer locations, with the most frequent being colorectal, breast, uterine, hematologic, and pancreatic cancers.123
Hormonal treatment may be continued during anticoagulant treatment in women who had experienced VTE to prevent pregnancy and mitigate the risk of abnormal uterine bleeding.124
Thrombophilia testing is not recommended in patients with VTE, and only should be performed if the results may modify the management of the patient in terms of type, dosage, or duration of anticoagulant therapy or counseling on oral contraception or prophylactic measures during pregnancy for female first-degree relatives.124
Pulmonary Arterial Hypertension (PAH)Registries of PAH have reported a higher incidence in women, with a female-to-male ratio ranging from 1.4:1 to 4.1:1.125,126 This difference between men and women is even greater in younger patients and tends to equalize after the age of 65.126 Female dominance has been associated with hormonal changes occurring during menopause.127 However, registries have shown that women with PAH have better survival rates than men. This difference between sexes in PAH has been referred to as the “estrogen paradox,” where although women have a higher susceptibility, once they develop the disease, they exhibit a better response to treatment and prognosis.128–130
Most of different subgroups of PAH have a similar distribution of sex. Women with connective tissue disease have a higher risk, up to 9 times, than men of developing PH.131,132 Up to 12% of women with systemic sclerosis will develop pulmonary hypertension, with this incidence being higher than in men, although survival is better.133,134 Other subgroups of PAH present a balanced distribution, with a slight predominance in men in amphetamine-induced PH and PH associated with HIV infection.135,136
A pooled analysis of randomized clinical trials that included 1130 patients with PAH receiving endothelin receptor antagonists or placebo concluded a better treatment response in women, measured by the 6-minute walking test (6MWT).137 On the other hand, a sub-analysis evaluating the response to tadalafil found that women had a worse quality of life and covered a shorter distance in the 6MWT.138 No differences have been found between sexes in the response to riociguat treatment.139 Among patients who required epoprostenol, women have fewer hospitalizations and better survival.140
When treatment adherence was analyzed, no differences were found between men and women.141
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)CTEPH is an infrequent complication of acute PE, and the diagnosis is based on findings obtained after at least 3 months of anticoagulation. These findings are mean pulmonary arterial pressure (mPAP)≥20mmHg with a pulmonary artery wedge pressure (PAWP)≤15mmHg measured in the right heart catheterization (RHC) and pulmonary vascular resistance (PVR)>2, with mismatched perfusion defects on lung scan and specific diagnostic signs for CTEPH seen by multidetector computed tomography pulmonary angiography (CTPA), magnetic resonance imaging (MRI) or conventional pulmonary cineangiography, such as ring-like stenoses, webs/slits and chronic total occlusions.142–144 The CTEPH treatment includes a global approach of combinations of pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), and medical therapies.143,144 The treatment of choice in these patients is PEA because it is a potentially curative treatment.
An observational study analyzed the incidence of risk factors for CTEPH, which included 23,329 patients with VTE, found that female sex was one of the variables associated with CTEPH.145 On the other hand, women with CTEPH have better survival,146,147 although with more hemodynamic deterioration than men.148 These findings can be justified by the fact that women undergo PEA less frequently and are classified more often as inoperable patients, especially in centers with a low patient volume.149 Another factor to consider is that women take longer to be diagnosed with CTEPH and therefore are in a more advanced clinical stage of the disease at the time of diagnosis.149 In a population-based cohort study, it was observed that there were no differences in survival between men and women undergoing PEA.150Fig. 1 summarizes the differences in vascular lung diseases between men and women.
ConclusionMen and women differ on the presentation and prognosis of respiratory diseases. Although women have been both under represented and under recognized in clinical practice and in studies, there's an increasing recognition of the influence of sex in airway diseases. Women with COPD experience more severe symptoms and exacerbations at younger ages, despite lower tobacco exposure, yet they remain underdiagnosed. In contrast, OSA is also underdiagnosed in women due to atypical symptoms and hormonal influences. Asthma prevalence and severity in women are influenced by hormonal changes, while bronchiectasis is more prevalent in women in Western populations, with unique clinical presentations influenced by hormonal and immune factors. Women are more prone to autoimmune-related ILD and experience delays in receiving treatments like antifibrotic therapy. PAH shows a higher prevalence but a better survival in women. There is a growing need to consider these factors in research and to personalize care and treatment based on sex and adapted to the different stages of a woman's life (Fig. 2).
Generative AIAI Involvement: some individual components of Fig. 2 were generated with the help of AI and then assembled together to create the finale figure.
FundingThis manuscript has not received any funding.
Conflicts of InterestLJP received grants from Leo Pharma and MSD and personal fees from Daichii, Rovi, GlaxoSmithKline, BMS and Johnson and Johnson outside the submitted work. No other conflict of interest declared.
MB has received speaker fees from Grifols, Menarini, CSL Behring, GSK, Boehringer Ingelheim, Chiesi and consulting fees from GSK, Novartis, Chiesi, CSL Behring, Chiesi and Boehringer Ingelheim.
MS received grants from GSK, Trudel medical int., and the Tel Aviv league for lung diseases, consulting fees from Astra Zeneca, Boehringer Ingelheim, Dexcel, Kamada, Synchrony medical, Truemed, Vertex, Zambon; speakers fees from Azstra Zeneca, Boehringer Ingelheim, GSK, Kamada, Sanofi, Insmed; participation in advisory and DSMB boradis- Bonus biotherapeutics, Astra Zeneca, Boehringer Ingelheim.
DA received grants from Boehringer-Ingleheim, Canadian Institute for Health Research, McGill University, and speakers fees and consulting fees from Hoffman La Roche and Boerhinger Ingelheim.
MH has received grants or contracts from NIH, Sanofi, Novartis, Nuvaira, Sunovion, Gala Therapeutics, COPD foundation, AstraZeneca, American Lung Association, Boehringer Ingelheim and Biodesix; consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Altesa BioPharma Amgen Roche, RS Biotherapeutics, ApregHealth, Genentech; speaker fees or honoraria from Cipla, Chiesi, AstraZeneca Boehringer Ingelheim, GSK, Medscape, integrity NACE, Medwiz.
FS has received grants form GSK and AZ, Honoraria for lectures: GSK, AZ, Chiesi, Sanofi, TEVA, ALK.
MP has received honoraria for lectures: Omron, Neopharmed Consultancy: Idorsia.