Right ventricle (RV) dysfunction increases the risk of death from pulmonary embolism (PE). C-reactive protein (CRP) might identify RV inflammation and dysfunction in patients with PE.
MethodsThis cohort study enrolled consecutive stable patients with acute PE between 2017 and 2023. We stratified patients by quartiles of CRP. We evaluated the association between CRP quartiles and the presence of RV dysfunction, and used multivariable models to assess for an association between CRP and the outcomes of all-cause and PE-specific mortality during the 30 days of follow-up after PE diagnosis.
ResultsThe study included 633 stable patients with PE. Patients without RV dysfunction had significantly lower median (IQR) CRP levels compared with patients with RV dysfunction (n=509, 31.7 [10.0–76.4]mg/L vs n=124, 45.4 [16.0–111.4]mg/L; P=0.018). CRP showed a statistically significant positive association with the presence of RV dysfunction (P<0.01). On multivariable analysis, CRP level was not significantly associated with 30-day all-cause mortality (adjusted odds ratio [OR] per mg/L increment, 1.00; 95% CI, 1.00–1.01; P=0.095), but higher CRP was associated with significantly higher PE-related mortality (adjusted OR, 1.01; 95% CI, 1.00–1.01; P=0.026). Compared with patients in CRP quartile 1, patients in quartiles 2, 3, and 4 had a stepwise increase in the adjusted odds of 30-day all-cause death of 2.41 (P=0.148), 3.04 (P=0.062), and 3.15 (P=0.052), respectively.
ConclusionsAs an indicator of RV dysfunction, CRP may improve risk stratification algorithms for hemodynamically stable patients with acute symptomatic PE.
In patients with acute symptomatic pulmonary embolism (PE), anticoagulation remains the primary treatment for the acute phase.1,2 However, the presence of right ventricle (RV) dysfunction increases the risk of death from PE, and studies have evaluated the efficacy and safety of thrombolytic therapy for these patients.3 Thrombolysis reduces clot burden faster than heparin, but is associated with an increased risk of major bleeding.4 Of note, approximately 16% of patients with PE have a contraindication to anticoagulation, and 30% have a contraindication to thrombolysis.5
Since the inflammatory response plays a primary role in the development of acute RV dysfunction during PE, it might represent a focus for treatments designed to alleviate the RV dysfunction aside from treatment that addresses thrombosis.6–9 However, in a randomized trial that enrolled 34 patients with intermediate-risk PE, treatment with diclofenac did not result in a significantly lower rate of RV dysfunction at 48h and 7 days in comparison to placebo.10 Besides the trial's small sample size, one of the underlying reasons for the lack of statistical significance in the intention-to-treat analysis might have been failure to identify the subgroup of patients with RV inflammation as the main cause for RV dysfunction.
Inflammation and tissue damage promote the production of C-reactive protein (CRP). Studies have shown an association between CRP level elevation and increased risk of cardiovascular and cerebrovascular events.11,12 For patients with acute symptomatic PE, systemic inflammation might help identify patients with RV dysfunction secondary to inflammation of the RV muscle who might benefit from anti-inflammatory therapy. Moreover, the prognostic value of CRP among patients with acute PE lacks clarity.13
Accordingly, we evaluated whether CRP level elevation in hemodynamically stable patients with acute PE assists with identifying those who have RV dysfunction. We also assessed whether CRP elevation is a risk factor for short-term all-cause and PE-related mortality among these patients.
MethodsStudy DesignIn a prospective cohort study, we approached consecutive eligible patients for enrollment between January 1, 2017, and May 31, 2023.
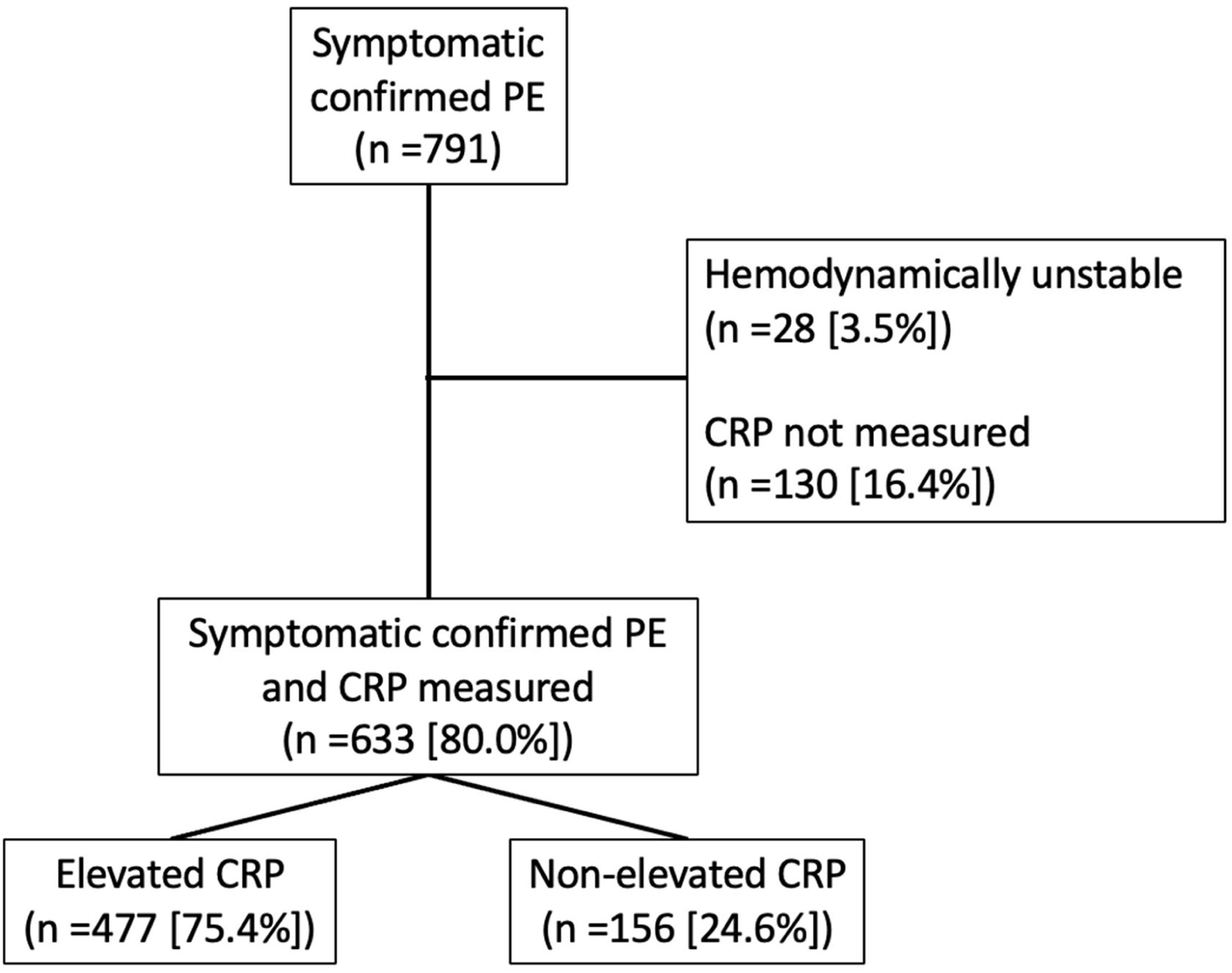
Patients, Setting, and Eligibility CriteriaWe screened outpatients presenting with symptoms of acute PE at the Emergency Department of Ramón y Cajal Hospital, Madrid, Spain (Fig. 1). Eligible patients were required to have symptomatic PE confirmed by objective testing. We excluded patients with a diagnosis of COVID-associated PE. Patients with haemodynamic instability (defined as cardiogenic shock, systolic blood pressure <90mmHg, or need of inotropic support), those in whom thrombolytic therapy was indicated by decision of attending physician, and those in whom CRP levels could not be determined within 24h from the diagnosis of PE, were also excluded. The study was approved by the Institutional Review Board of Ramón y Cajal Hospital.
Diagnosis of PEFor the diagnosis of PE, the study required the finding of an intraluminal filling defect on pulmonary embolism protocol contrast-enhanced helical chest computed tomography,14 a high probability ventilation–perfusion scan according to the criteria of the Prospective Investigation of Pulmonary Embolism Diagnosis,15 or an indeterminate ventilation–perfusion lung scan associated with a confirmed lower limb (proximal or distal) deep vein thrombosis on venous compression ultrasonography.16
C-reactive Protein AssayFrom blood drawn in a serum gel tube, we analyzed serum for CRP. We employed a conventional particle-enhanced nephelometry to determine CRP levels (Dimension Vista, Siemens, Berlin, Germany). This system measured concentrations from 2.9 to 290mg/L.17 For higher concentrations, we diluted samples 2-fold and reanalyzed them.
Echocardiography AssessmentPatients underwent transthoracic echocardiography within 24h after diagnosis of PE. Trained and certified local cardiologists, blinded to the patient's clinical data, interpreted each echocardiogram based on guideline recommendations.18 The study defined echocardiographic RV dysfunction as the presence of at least two of the following criteria: (i) RV diastolic diameter at the midlevel (within the chamber) >30mm in the parasternal window; (ii) RV diameter at the midlevel greater than that of the left ventricle in the apical or subcostal space; (iii) RV free wall hypokinesis; and (iv) estimated pulmonary artery systolic pressure >30mmHg.19
Follow-up and Outcome AssessmentPrognostic test results did not mandate treatment options. In the absence of an absolute contraindication to anticoagulation, clinicians-initiated treatment with parenteral anticoagulation combined with oral vitamin-K antagonist therapy, or with direct oral anticoagulants. In patients whose clinical status deteriorated after enrollment, clinicians administered reperfusion treatment and/or inotropic support as deemed appropriate.
We used transthoracic echocardiographic RV dysfunction within 24h after diagnosis of PE as the study's primary outcome. For 30 days after the diagnosis of PE, we also evaluated patients for all-cause and PE-related death. Two independent experts (authors C.R. and D.J.), blinded to the CRP results, classified the cause of death as PE-related if1 autopsy deemed PE as the cause of death, or2 death was most likely due to a PE-related deterioration or an objectively confirmed recurrent event, and most likely not due to another cause.
Statistical AnalysisData are presented as mean±SD or as median (IQR), as appropriate, for continuous variables and frequencies for categorical variables. Patients were divided into quartiles of CRP, with the lowest quartile (i.e., CRP<11.0mg/L) as the reference category. Quartiles were chosen to ensure a similar number of patients in each CRP category. We first conducted bivariable analysis comparing the demographic and clinical characteristics of patients across the quartiles of CRP with analysis of variance or Kruskal–Wallis test for continuous variables, and the chi-square test for categorical variables.
Association between CRP quartiles and the presence of RV dysfunction were determined using the Kendall's tau-b correlation coefficient. To determine CRP's diagnostic accuracy for echocardiographic RV dysfunction, we used receiver operating characteristic curves and analyzed area under the curves (AUC).
To assess the relationship between CRP and the outcomes of interest, we constructed multivariable logistic regression models for the overall cohort. We addressed potential confounding by controlling for the severity of illness and additional variables related to the outcome. The following models were generated sequentially to determine the successive influence of potential confounders on the relationship between CRP levels and mortality1: unadjusted2; adjusted only for age (as a continuous variable) and sex; and3 adjusted for age (as a continuous variable), sex, and the severity of PE (assessed by the simplified Pulmonary Embolism Severity Index [sPESI]). All these models were built with CRP as a continuous variable, and as a 4-category predictor variable.
We assessed the sensitivity of our findings by repeating the primary analysis under varying assumptions. Sensitivity analyses comprised comparing outcomes between those with normal and those with elevated CRP levels, using cut-off levels of <10mg/L and <5mg/L for a normal CRP.20 Since active cancer may produce an inflammatory response that elevated the CRP level,21 we also repeated analyses after excluding patients who had an active cancer (n=90).
We conducted analyses using SPSS software version 29.0.1.0 and considered a two-sided P value of <0.05 significant.
ResultsPatients and Baseline CharacteristicsIn the Emergency Department, we screened 791 consecutive patients diagnosed with non-COVID-19-associated acute symptomatic PE. We excluded 28 (3.5%) patients because of haemodynamic instability and an additional 130 patients (16.4%) because of CRP level not being determined within 24h from the diagnosis of PE. We enrolled the remaining eligible 633 patients (297 men and 336 women) (Fig. 1).
The cohort had a median CRP concentration of 34.0mg/L (IQR 11.0–84.2mg/L; distribution shown in Supplemental Fig. 1). Of the 633 enrolled patients, 477 (75.4%) had an elevated CRP level (i.e., >10mg/L) (CRP-positive group) and the remaining 156 (24.6%) patients had a normal serum CRP level (i.e., <10mg/L) (CRP-negative group).
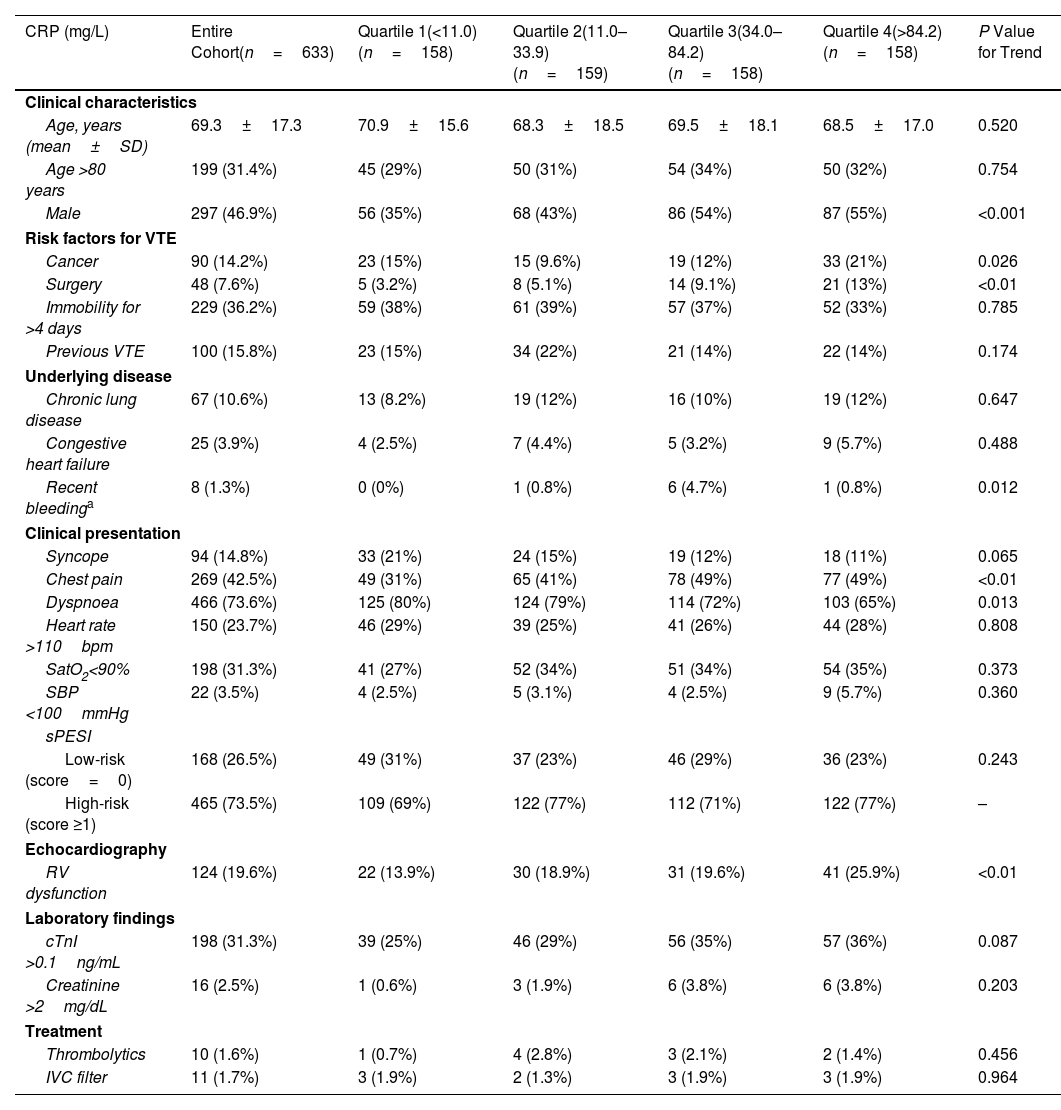
Relationship Between CRP Levels and Baseline CharacteristicsTable 1 describes baseline characteristics stratified by quartiles of CRP. The CRP quartiles had similar mean age and vital signs (i.e., heart rate, oxygen saturation, and systolic blood pressure), and similar frequencies of immobilization, history of previous VTE, and comorbidities (i.e., chronic obstructive disease and congestive heart failure) at presentation. Patients in the higher quartiles were more likely to have male sex, history of cancer, previous surgery, and recent bleeding than patients in the lower CRP quartiles. Dyspnoea occurred more frequently in the lower CRP quartiles while chest pain occurred more frequently in the higher quartiles. The CRP quartiles did not have significantly different rates of treatment with a thrombolytic agent or insertion of an inferior vena cava filter during follow-up (Table 1).
Baseline Characteristics of the Study Cohort Divided by CRP Quartile.
| CRP (mg/L) | Entire Cohort(n=633) | Quartile 1(<11.0) (n=158) | Quartile 2(11.0–33.9) (n=159) | Quartile 3(34.0–84.2) (n=158) | Quartile 4(>84.2) (n=158) | P Value for Trend |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age, years (mean±SD) | 69.3±17.3 | 70.9±15.6 | 68.3±18.5 | 69.5±18.1 | 68.5±17.0 | 0.520 |
| Age >80 years | 199 (31.4%) | 45 (29%) | 50 (31%) | 54 (34%) | 50 (32%) | 0.754 |
| Male | 297 (46.9%) | 56 (35%) | 68 (43%) | 86 (54%) | 87 (55%) | <0.001 |
| Risk factors for VTE | ||||||
| Cancer | 90 (14.2%) | 23 (15%) | 15 (9.6%) | 19 (12%) | 33 (21%) | 0.026 |
| Surgery | 48 (7.6%) | 5 (3.2%) | 8 (5.1%) | 14 (9.1%) | 21 (13%) | <0.01 |
| Immobility for >4 days | 229 (36.2%) | 59 (38%) | 61 (39%) | 57 (37%) | 52 (33%) | 0.785 |
| Previous VTE | 100 (15.8%) | 23 (15%) | 34 (22%) | 21 (14%) | 22 (14%) | 0.174 |
| Underlying disease | ||||||
| Chronic lung disease | 67 (10.6%) | 13 (8.2%) | 19 (12%) | 16 (10%) | 19 (12%) | 0.647 |
| Congestive heart failure | 25 (3.9%) | 4 (2.5%) | 7 (4.4%) | 5 (3.2%) | 9 (5.7%) | 0.488 |
| Recent bleedinga | 8 (1.3%) | 0 (0%) | 1 (0.8%) | 6 (4.7%) | 1 (0.8%) | 0.012 |
| Clinical presentation | ||||||
| Syncope | 94 (14.8%) | 33 (21%) | 24 (15%) | 19 (12%) | 18 (11%) | 0.065 |
| Chest pain | 269 (42.5%) | 49 (31%) | 65 (41%) | 78 (49%) | 77 (49%) | <0.01 |
| Dyspnoea | 466 (73.6%) | 125 (80%) | 124 (79%) | 114 (72%) | 103 (65%) | 0.013 |
| Heart rate >110bpm | 150 (23.7%) | 46 (29%) | 39 (25%) | 41 (26%) | 44 (28%) | 0.808 |
| SatO2<90% | 198 (31.3%) | 41 (27%) | 52 (34%) | 51 (34%) | 54 (35%) | 0.373 |
| SBP <100mmHg | 22 (3.5%) | 4 (2.5%) | 5 (3.1%) | 4 (2.5%) | 9 (5.7%) | 0.360 |
| sPESI | ||||||
| Low-risk (score=0) | 168 (26.5%) | 49 (31%) | 37 (23%) | 46 (29%) | 36 (23%) | 0.243 |
| High-risk (score ≥1) | 465 (73.5%) | 109 (69%) | 122 (77%) | 112 (71%) | 122 (77%) | – |
| Echocardiography | ||||||
| RV dysfunction | 124 (19.6%) | 22 (13.9%) | 30 (18.9%) | 31 (19.6%) | 41 (25.9%) | <0.01 |
| Laboratory findings | ||||||
| cTnI >0.1ng/mL | 198 (31.3%) | 39 (25%) | 46 (29%) | 56 (35%) | 57 (36%) | 0.087 |
| Creatinine >2mg/dL | 16 (2.5%) | 1 (0.6%) | 3 (1.9%) | 6 (3.8%) | 6 (3.8%) | 0.203 |
| Treatment | ||||||
| Thrombolytics | 10 (1.6%) | 1 (0.7%) | 4 (2.8%) | 3 (2.1%) | 2 (1.4%) | 0.456 |
| IVC filter | 11 (1.7%) | 3 (1.9%) | 2 (1.3%) | 3 (1.9%) | 3 (1.9%) | 0.964 |
Abbreviations: CRP, C-reactive protein; VTE, venous thromboembolism; SBP, systolic blood pressure; sPESI, simplified Pulmonary Embolism Severity Index; cTnI, cardiac troponin I; IVC, inferior vein cava.
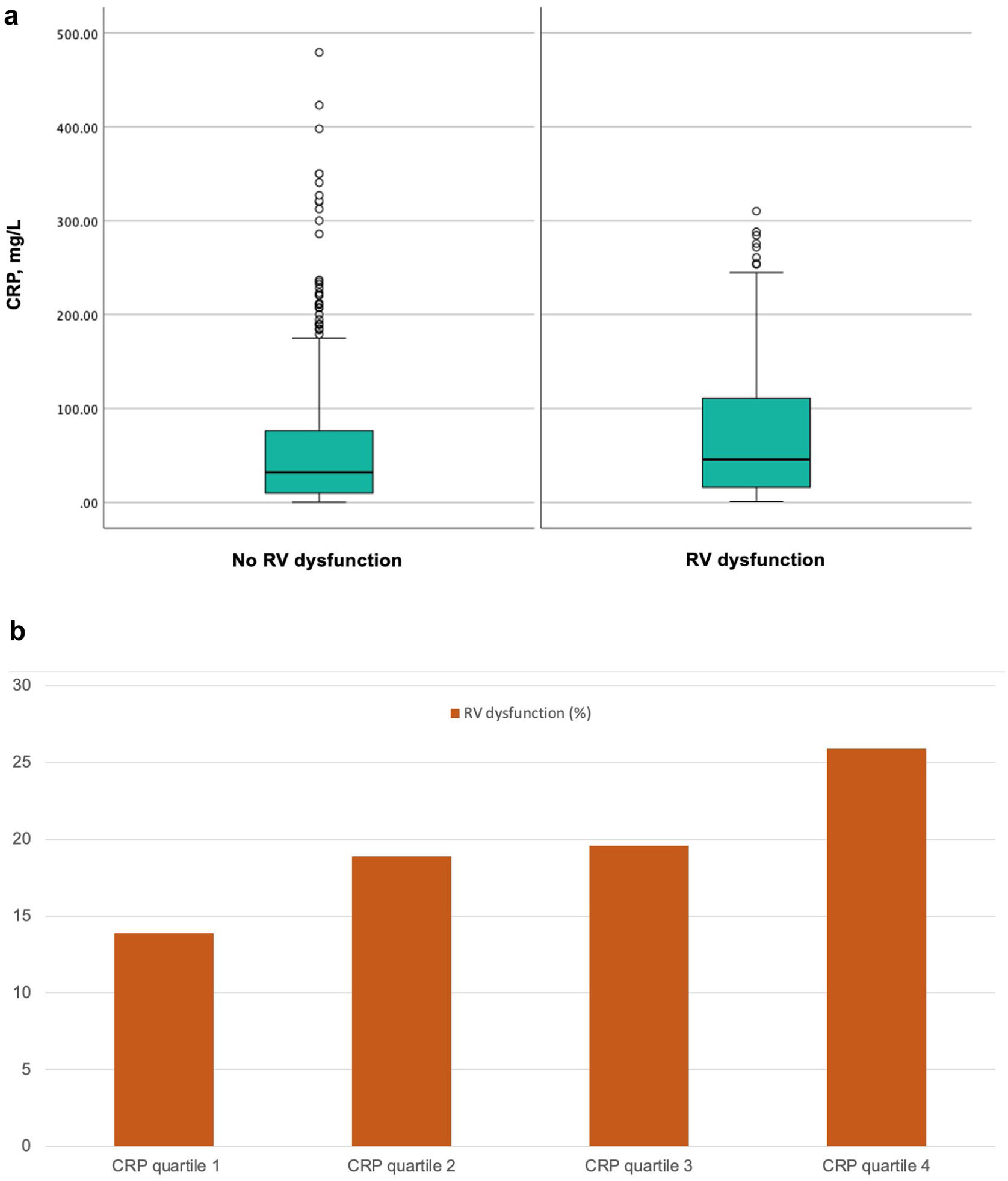
One hundred and twenty-four (19.6%) patients met criteria for echocardiographic RV dysfunction (within 24h of PE diagnosis). Patients without RV dysfunction had significantly lower CRP levels compared with patients with RV dysfunction (31.7 [10.0–76.4]mg/L vs 45.4 [16.0–111.4]mg/L; P=0.018) (Fig. 2a). CRP quartile was significantly associated with RV dysfunction (Kendall's tau-b correlation coefficient, 0.095; P<0.01) with increasing quartiles showing a greater frequency of RV dysfunction (Fig. 2b). CRP (as a continuous variable) marginally discriminated between patients with and those without RV dysfunction (AUC, 0.58).
(a) Box plots with serum concentrations of C-reactive protein according to the presence or absence of right ventricle dysfunction. (b) Distribution of patients with right ventricle dysfunction among C-reactive protein quartiles in patients with pulmonary embolism (Kendall's tau-b correlation coefficient, 0.095; P<0.01). Abbreviations: CRP, C-reactive protein; RV, right ventricle.
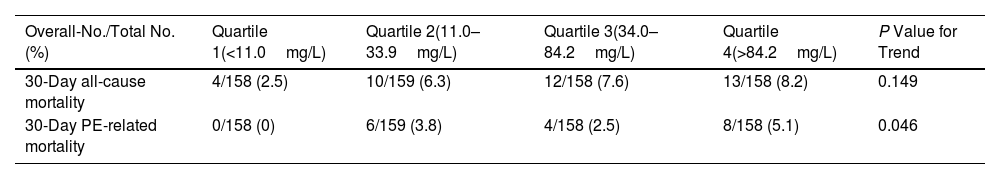
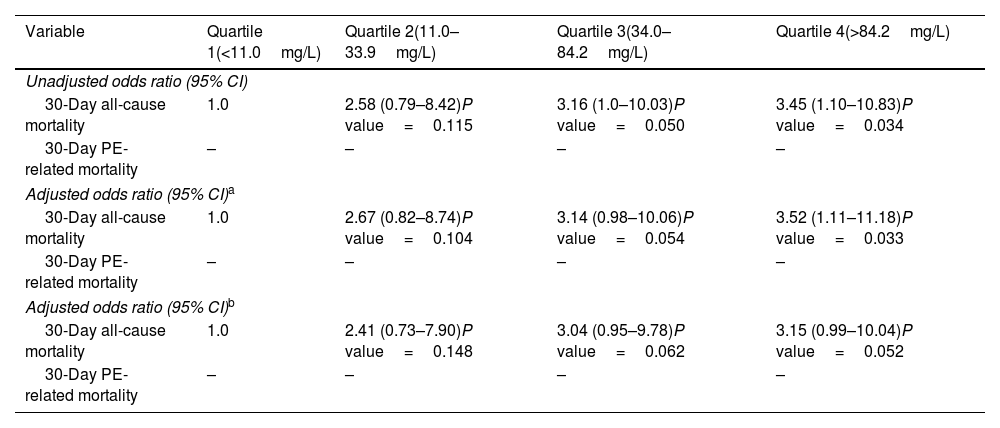
The study had complete clinical outcome information for all patients at the end of the 30-day follow-up. The entire cohort had a 30-day all-cause mortality of 6.2% (39 of 633 patients), and a 30-day PE-related mortality rate of 2.8% (18 of 633 patients) (Table 2). Death unrelated to PE was caused by cancer in 10 (1.6%) patients, major bleeding in 3 (0.5%) patients, and unknown or other causes in 8 (1.3%) patients. When examined as a continuous variable (between 0.2 and 479.4mg/L), CRP level was not associated with 30-day all-cause mortality (unadjusted odds ratio [OR], 1.00; 95% CI, 1.00–1.01; P=0.095), but it was associated with 1% higher odds of PE-related death per 1mg/L increase (unadjusted OR, 1.01; 95% CI, 1.00–1.01; P=0.026). On univariable analysis, as compared with patients in the lowest quartile (quartile 1) of CRP, patients in quartiles 2, 3, and 4 had an increase in the odds of 30-day all-cause death of 2.58 (P=0.115), 3.16 (P=0.050), and 3.45 (P=0.034), respectively (Table 3).
Observed Rates of Mortality by CRP Quartile.
| Overall-No./Total No. (%) | Quartile 1(<11.0mg/L) | Quartile 2(11.0–33.9mg/L) | Quartile 3(34.0–84.2mg/L) | Quartile 4(>84.2mg/L) | P Value for Trend |
|---|---|---|---|---|---|
| 30-Day all-cause mortality | 4/158 (2.5) | 10/159 (6.3) | 12/158 (7.6) | 13/158 (8.2) | 0.149 |
| 30-Day PE-related mortality | 0/158 (0) | 6/159 (3.8) | 4/158 (2.5) | 8/158 (5.1) | 0.046 |
Abbreviation: CRP, C-reactive protein.
Adjusted Rates of Mortality by CRP Quartile.
| Variable | Quartile 1(<11.0mg/L) | Quartile 2(11.0–33.9mg/L) | Quartile 3(34.0–84.2mg/L) | Quartile 4(>84.2mg/L) |
|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | ||||
| 30-Day all-cause mortality | 1.0 | 2.58 (0.79–8.42)P value=0.115 | 3.16 (1.0–10.03)P value=0.050 | 3.45 (1.10–10.83)P value=0.034 |
| 30-Day PE-related mortality | – | – | – | – |
| Adjusted odds ratio (95% CI)a | ||||
| 30-Day all-cause mortality | 1.0 | 2.67 (0.82–8.74)P value=0.104 | 3.14 (0.98–10.06)P value=0.054 | 3.52 (1.11–11.18)P value=0.033 |
| 30-Day PE-related mortality | – | – | – | – |
| Adjusted odds ratio (95% CI)b | ||||
| 30-Day all-cause mortality | 1.0 | 2.41 (0.73–7.90)P value=0.148 | 3.04 (0.95–9.78)P value=0.062 | 3.15 (0.99–10.04)P value=0.052 |
| 30-Day PE-related mortality | – | – | – | – |
Abbreviations: CI, confidence interval; PE, pulmonary embolism; VTE, venous thromboembolism.
There were no PE-related deaths in quartile 1 of CRP and odds ratios could not be calculated.
Adjusted 30-day all-cause mortality was 2.5% for patients with CRP levels in the lowest quartile and 8.2% for patients with levels in the highest quartile. Similar findings were observed for 30-day PE-related mortality. The adjusted risk of 30-day PE-related death was 0% for patients in the lowest quartile of CRP levels and 5.1% for patients in the highest quartile of CRP levels.
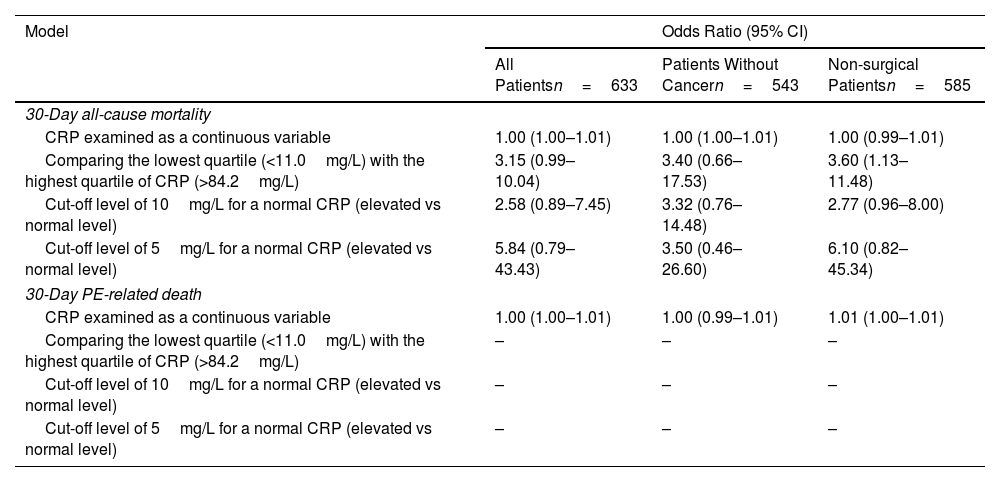
SubanalysesTo explore the sensitivity of our findings, we repeated the analysis with varying assumptions (Table 4). The use of a cut-off level of 10mg/L or 5mg/L for a normal CRP did not significantly change the main findings. After excluding patients with cancer, we found similar results, with a significant association between CRP quartile and the presence of RV dysfunction (Kendall's tau-b correlation coefficient, 0.105; P<0.01).
Sensitivity and Subgroup Analyses for Mortality Rates.*
| Model | Odds Ratio (95% CI) | ||
|---|---|---|---|
| All Patientsn=633 | Patients Without Cancern=543 | Non-surgical Patientsn=585 | |
| 30-Day all-cause mortality | |||
| CRP examined as a continuous variable | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.01) |
| Comparing the lowest quartile (<11.0mg/L) with the highest quartile of CRP (>84.2mg/L) | 3.15 (0.99–10.04) | 3.40 (0.66–17.53) | 3.60 (1.13–11.48) |
| Cut-off level of 10mg/L for a normal CRP (elevated vs normal level) | 2.58 (0.89–7.45) | 3.32 (0.76–14.48) | 2.77 (0.96–8.00) |
| Cut-off level of 5mg/L for a normal CRP (elevated vs normal level) | 5.84 (0.79–43.43) | 3.50 (0.46–26.60) | 6.10 (0.82–45.34) |
| 30-Day PE-related death | |||
| CRP examined as a continuous variable | 1.00 (1.00–1.01) | 1.00 (0.99–1.01) | 1.01 (1.00–1.01) |
| Comparing the lowest quartile (<11.0mg/L) with the highest quartile of CRP (>84.2mg/L) | – | – | – |
| Cut-off level of 10mg/L for a normal CRP (elevated vs normal level) | – | – | – |
| Cut-off level of 5mg/L for a normal CRP (elevated vs normal level) | – | – | – |
There were no PE-related deaths in quartile 1 of CRP, and odds ratios could not be calculated.
In this prospective observational cohort study of 633 patients with haemodynamically stable acute symptomatic PE diagnosed in the Emergency Department, higher CRP was significantly associated with the presence of echocardiographic RV dysfunction. We also found a significant association between CRP level and risk-adjusted 30-day PE-related mortality. Sensitivity and subgroup analyses showed similar findings.
Though some data have suggested a positive relationship between increased CRP concentration and venous thromboembolism incidence,22 limited information exists about the role of CRP as a marker of RV inflammation and dysfunction. This study's findings expand on those from a previous study of 56 patients with acute symptomatic PE,13 where RV dysfunction occurred more frequently in patients with higher CRP levels (P=0.020). Mortality rates were higher in patients with elevated CRP levels (not statistically significant).
The current study shows that a single measurement of CRP is strongly associated with the presence of RV dysfunction in acute PE. However, whether CRP itself is truly part of the causal pathway between inflammation and RV dysfunction, or may only be indicative of a generalized inflammatory state, remains uncertain. Our analysis cannot determine the direction of the association (i.e., definitive conclusions on causality cannot be made). Therefore, we consider the results of this cohort study as hypothesis-generating, and we do not recommend modification of the current approach to risk stratification of patients with acute symptomatic PE.23 Since higher CRP levels may indicate a higher extent of cardiac inflammation, and more susceptibility to RV dysfunction, our data may inform the design of randomized trials evaluating the efficacy and safety of anti-inflammatory drugs for patients with acute symptomatic PE.
Several potential limitations of our study merit consideration. This hypothesis-generating study was underpowered for detecting a relationship between CRP level and 30-day all-cause mortality, and some results may be indicative of a type II error. Despite our best efforts, the possibility of residual confounding remains. Nevertheless, after adjusting for age, and severity of PE, we still found a consistent CRP level-outcome relationship. We used a single measurement of CRP, while concentrations might fluctuate even during the acute phase of PE. However, based on current guidelines and the published literature, prognostication of PE is based on the findings at the time of PE diagnosis. We did not measure other inflammatory markers such as IL-6, neutrophil-to-lymphocyte-ratio, platelet-to-lymphocyte-ratio or systemic-immune-inflammation-index (SII).24 While different groups of investigators have not agreed upon standard definitions for echocardiographic findings of RV dysfunction, the study used a set of criteria that have been commonly employed in the medical literature.19 Finally, we did not collect detailed data on auto-inflammatory and infectious diseases that may increase CRP, or prior use of statins that may decrease CRP. However, the sensitivity and subgroup analyses suggest that those conditions may have limited influence on the results of this study.
In conclusion, we observed an association between CRP level and echocardiographic RV dysfunction and mortality among hemodynamically stable patients with acute symptomatic PE. Additional research may confirm these results, and may further support the investigation of adjunct anti-inflammatory therapies for PE patients who have evidence of systemic inflammation and RV dysfunction.
Authors’ ContributionsStudy concept and design: Najarro, Bikdeli, Jimenez.
Acquisition of data; analysis and interpretation of data; statistical analysis: Najarro, Rodríguez, Morillo, Jara-Palomares, Vinson, Muriel, Álvarez-Mon, Yusen, Bikdeli, Jimenez.
Drafting of the manuscript: Yusen, Bikdeli, Jiménez.
Critical revision of the manuscript for important intellectual content: Najarro, Rodríguez, Morillo, Jara-Palomares, Vinson, Muriel, Álvarez-Mon, Yusen, Bikdeli, Jimenez.
Study supervision: Jimenez.
FundingNone declared.
Conflict of InterestsThe authors state that they have no conflict of interests.