In patients with acute symptomatic pulmonary embolism (PE), embolic burden has an uncertain prognostic significance.
MethodsWe performed a meta-analysis of studies including patients with PE to assess the prevalence and prognostic relevance of central PE (i.e., saddle or main pulmonary emboli) for short-term death and other adverse outcome events. A random-effects model was used to pool study results, and I2 testing was used to test for heterogeneity.
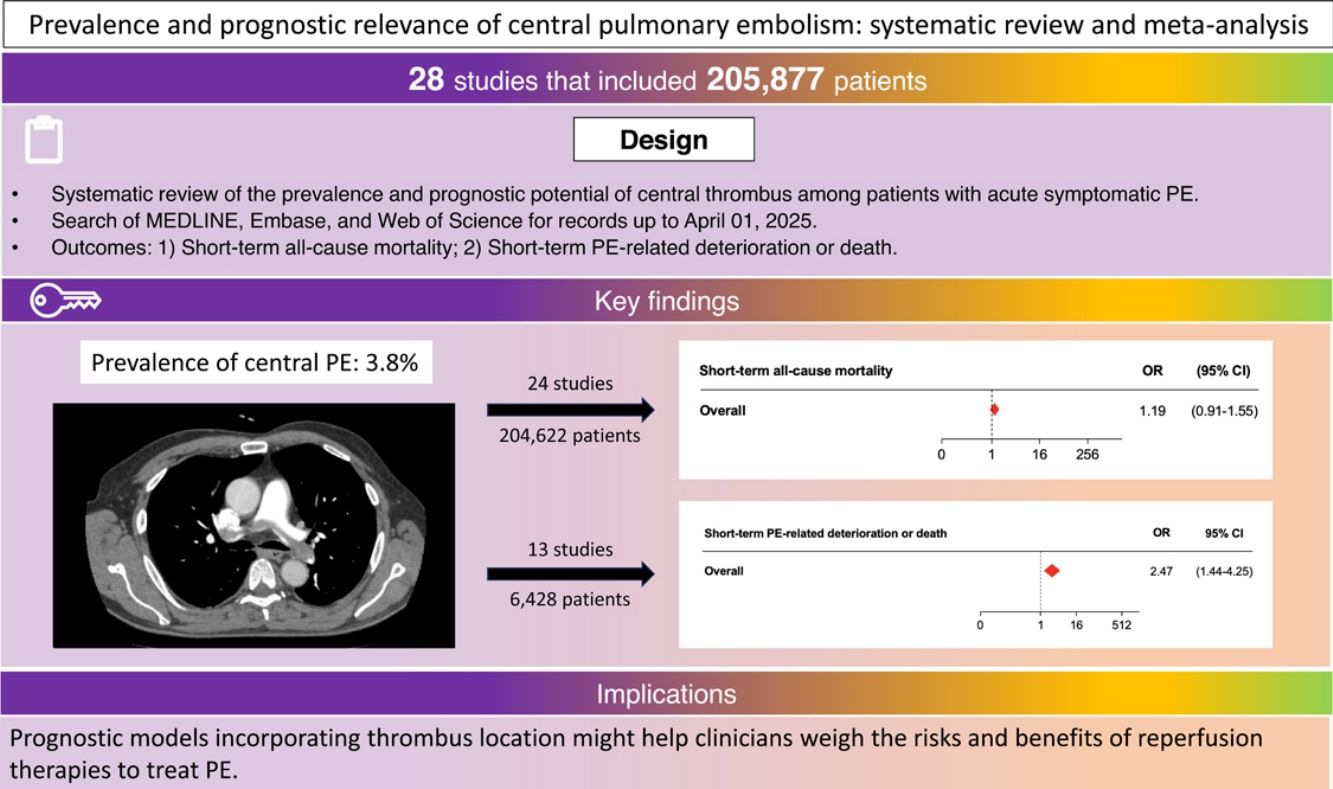
ResultsData from 28 studies (205,877 patients) were included in the analysis. Of the 26 studies with 205,410 participants that enrolled consecutive patients with PE, 7748 (3.8%) had central PE. Though there was no statistically significant difference between central and noncentral PE for odds of short-term all-cause mortality (odds ratio [OR] 1.19; 95% confidence interval [CI], 0.91–1.55), central PE had a significant association with short-term PE-related deterioration or death (OR, 2.47; 95% CI, 1.44–4.25). Results from 9 studies with 4325 PE patients that had available data showed that central PE had a significant association with the odds of short-term PE-related mortality (OR, 1.77; 95% CI, 1.22–2.55). Results were consistent for prospective (OR, 1.67; 95% CI, 1.21–2.32), and studies that only enrolled stable patients (OR, 1.42; 95% CI, 1.06–1.92).
ConclusionsCentral PE was an uncommon finding in unselected patients diagnosed with PE. While central thrombus did not have an association with increased risk of all-cause mortality, it was significantly associated with an increased risk of PE-related deterioration and death short after diagnosis.
Pulmonary embolism (PE) can be life-threatening if not diagnosed and treated quickly [1]. One of the critical pillars for the management of acute PE is adequate risk stratification, as it may influence decisions for more intensive vigilance and escalation of treatment (i.e., reperfusion therapy) beyond anticoagulation [2,3].
Although life-threatening PE has been equated with anatomically massive PE (defined as a >50% obstruction of the pulmonary vasculature or the occlusion of two or more lobar arteries) [4–6], it seems reasonable to propose that the outcome from PE is a function of both the size of the embolus and the underlying multiorgan reserve, particularly cardiopulmonary function [7]. Therefore, actual risk stratification models have replaced the burden of embolic obstruction with hemodynamic status, and presence/absence of right ventricle (RV) dysfunction on imaging studies and plasma biomarkers of myocardial injury [8]. However, central thrombus burden (i.e., saddle PE and/or PE in the main pulmonary arteries) might still portend worse outcomes, and may help with risk classification of patients with acute symptomatic PE [9,10]. Moreover, even in the absence of guideline-endorsed recommendations, the presence of central thrombus often guide use of mechanical thrombectomy [9], and confirmation of the prognostic relevance of central PE might help with clinical decision-making.
Though the exact occurrence is unknown, studies of patients with proven acute PE have reported a high prevalence of central PE of up to 40% [11]. However, studies have shown conflicting data regarding the association between central PEs and short-term mortality. While some studies found the presence of central PE to be an independent predictor of adverse patient outcomes [12], others did not confirm these findings [13]. To address this discrepancy, and since the presence of central thrombus can be easily assessed at computed tomography (CT) angiography, it is key to evaluate its role in the prognostic stratification of patients with acute symptomatic PE.
To clarify the prevalence and prognostic potential of central thrombus among patients with acute symptomatic PE and its usefulness for treatment decision-making, this study aimed to review the literature systematically and perform an updated meta-analysis.
MethodsWe submitted the systematic review protocol for registration on PROSPERO (CRD420251061557). We have followed the Reporting Checklist for Meta-analyses of Observational Studies (MOOSE) [14] and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [15] to conduct and report this systematic review.
Study objectivesThe study aimed to assess for an association between central PE (i.e., saddle PE and/or PE in the main pulmonary arteries) and short-term (i.e., through 30 days after the diagnosis of PE) all-cause mortality in patients with acute symptomatic PE. The study also aimed to assess for an association between central PE and short-term PE-related adverse outcomes (i.e., clinical deterioration and/or PE-related death).
Data sources and searchesWe searched MEDLINE (using the Ovid platform), Embase (Elsevier), and Web of Science from inception through April 01, 2025. The search strategy combined terms for (i) PE, (ii) thrombus location, and (iii) prediction, risk, or prognosis (eTable 1) [16]. We did not limit our search by language. Full articles of all potentially appropriate abstracts were reviewed. Manual search of cited bibliographies and investigator files complemented the literature search.
Inclusion and exclusion criteriaCohort (prospective or retrospective) and cross-sectional studies (to assess the prevalence of central PE); and cohort and case-control studies (to assess for an association between central PE and clinical outcomes) were eligible if they (1) were conducted among adults with an objective diagnosis of PE; (2) provided information on CT-assessed localization of the thrombus; and (3) enrolled consecutive patients (i.e. an inception cohort or a retrospective identification of consecutive patients). Studies were included if they reported all-cause or PE-related adverse outcomes up to 30-days after PE diagnosis. For duplicate publications, the most recent was considered. We excluded studies that enrolled patients with nonthrombotic PE, such as fat or air embolism.
Study selectionTwo investigators (W.B. and G.D.) independently assessed identified articles to determine study eligibility. Based on title and abstract review, the reviewers excluded non-relevant studies. For relevant studies, the reviewers independently carried out data extraction using a pre-piloted, standardized form. Consensus or discussion with a third reviewer (D.J.) resolved eligibility and data extraction discrepancies or uncertainties.
Data extraction and quality assessmentFor each study, investigators abstracted data regarding study design (number of included patients; prospective or retrospective; single-center or multicenter), patient characteristics (age, sex, and hemodynamic status at inclusion in the study), CT testing results (percentage of patients with central PE) and outcomes. Two investigators (W.B. and D.J.) used the Quality in Prognosis Studies (QUIPS) tool to independently assess the quality of the eligible studies [17].
Data synthesis and analysisFor each study, we determined the pooled incidence of 30-day all-cause mortality, and 30-day PE-related deterioration or death for patients with versus those without CT-determined RV central PE. We calculated an odds ratio and the 95% confidence interval (CI) for the point estimates and pooled the odds ratios across studies by using a random-effects model approach. Statistical heterogeneity between groups was measured using the Cochran's Q statistic and the Higgins I2 statistic [18]. The Begg rank correlation method assessed for publication bias.
Pre-specified subgroup sensitivity analysesSubgroup sensitivity analyses were conducted to explore potential sources of heterogeneity. A planned exploratory analysis involved the study design (i.e., prospective vs. retrospective cohort studies). Also, we analyzed data for subgroup effects by hemodynamic status (i.e., only hemodynamically stable patients vs. stable and unstable patients) and central PE type (i.e., saddle PE). We ran supplemental analyses with inverse variance fixed-effects models. We also repeated separate analyses with short-term PE-related mortality and short-term PE-related complications as the outcome events. Finally, we explored the prognostic significance of central PE among those studies that assessed in-hospital mortality.
All analyses were carried out using IBM SPSS software, version 29.0 for Mac (SPSS, Inc. Chicago, IL, USA).
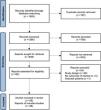
ResultsDescription of studiesWe initially retrieved a total of 1583 articles from various databases. After screening titles and abstracts, we excluded 187 duplicate articles and 758 articles irrelevant to the research objectives. After a detailed screening process, we finally included 28 articles [9,11–13,19–42] involving 205,877 participants in the analysis (Fig. 1 and Table 1).
Characteristics of the studies included.
| Author | Year | Study design | Main outcome event | Central PE, % | Population, n | Sex | Age, mean (SD) or range, y | Follow-up | Study results |
|---|---|---|---|---|---|---|---|---|---|
| Pruszczyk (19) | 2003 | Retrospective cohort | All-cause mortality | 27.9 | 61 | 41% female | 66 (11) | Two-weeks | 5.9% mortality rate for central PE group vs 36.4% for noncentral PE group |
| Kaczynska (20) | 2005 | Retrospective cohort | All-cause mortality | 14.7 | 150 | 63% female | 63.6+16.7 | In-hospital | 4.6% mortality rate for central PE group vs 13.3% for noncentral PE group |
| Ryu (22) | 2007 | Retrospective cohort | All-cause mortality | 2.6 | 546 | NA | NA | In-hospital | None of the patients died during their hospitalization |
| Ghanima (21) | 2007 | Retrospective cohort | All-cause mortality | 53.5 | 99 | NA | NA | 30-days | 5.7% mortality rate for central PE group vs 2.2% for noncentral PE group |
| Ozsu (41) | 2010 | Prospective cohort | All-cause mortality | 31.5 | 108 | 56% female | 70 (21–90) | 30-days | 14.7% mortality rate for central PE group vs 12.2% for noncentral PE group |
| Gandara (23) | 2011 | Case-control | All-cause mortality | –a | 93 | NA | 61 (16) | 30-days | 6.5% mortality rate for central PE group vs 9.7% for noncentral PE group |
| Vedovati (25) | 2012 | Prospective cohort | All-cause mortality | 60.1 | 579 | 52% female | 68.5+16.3 | 30-days | 6.3% mortality rate for central PE group vs 5.2% for noncentral PE group |
| Furlan (24) | 2012 | Retrospective cohort | All-cause mortality | 28.7 | 635 | 52% female | Male: 58+16Female: 61+18 | 30-days | 5.5% mortality rate for central PE group vs 6.4% for noncentral PE group |
| Park (42) | 2012 | Retrospective cohort | All-cause mortality | 69.6 | 56 | 50% female | 63.5 (52–71) | 30-days | 33.3% mortality rate for central PE group vs 5.9% for noncentral PE group |
| Kwak (26) | 2013 | Retrospective cohort | All-cause mortality | 9.1 | 297 | 52% female | Saddle: 62.5+12.1Non-saddle: 62.1+15.4 | 30-days | 18.5% mortality rate for central PE group vs 11.9% for noncentral PE group |
| Choi (27) | 2014 | Retrospective cohort | All-cause mortality | 35.5 | 743 | NA | NA | In-hospital | 9.1% mortality rate for central PE group vs 6.7% for noncentral PE group |
| Pathak (28) | 2015 | Retrospective cohort | All-cause mortality | 0.8 | 187,762 | NA | NA | In-hospital | 3.6% mortality rate for central PE group vs 3.1% for noncentral PE group |
| Alonso-Martinez (29) | 2016 | Prospective cohort | All-cause mortality | 48.1 | 530 | 55% female | 76 (16) | 30-days | 6% mortality rate for central PE group vs 4% for noncentral PE group |
| Senturk (11) | 2017 | Prospective cohort | All-cause mortality | 36.5 | 874 | 54% female | 67 (19–96) | 30-days | 11.9% mortality rate for central PE group vs 6.5% for noncentral PE group |
| Gouin (30) | 2017 | Retrospective cohort | All-cause mortality | 40.5 | 6674 | 53% female | 66 | 30-days | 2.8% mortality rate for central PE group vs 2.6% for noncentral PE group |
| Alkinj (31) | 2017 | Case-control | All-cause mortality | –a | 374 | 50% female | 66 (56–75) | In-hospital | 4.3% mortality rate for central PE group vs 5.4% for noncentral PE group |
| Jain (9) | 2017 | Retrospective cohort | All-cause mortality | 63.9 | 269 | 49% female | 62.6+15.3 | 30-days | 9.3% mortality rate for central PE group vs 18.5% for noncentral PE group |
| Hajizadeh (33) | 2019 | Retrospective cohort | All-cause mortality | 14.2 | 492 | 48% female | 61.9+17.2 | In-hospital | 28.6% mortality rate for central PE group vs 6.2% for noncentral PE group |
| Akhoundi (32) | 2019 | Retrospective cohort | All-cause mortality | 2.9 | 104 | 50% female | 63 (48–74) | 30-days | 0% mortality rate for central PE group vs 9.9% for noncentral PE group |
| Jia (13) | 2021 | Retrospective cohort | Deterioration, including PE-related shock, need for mechanical ventilation and cardiopulmonary resuscitation, life-saving hemodynamic support, and thrombolysis | 24.5 | 727 | NA | NA | 30-days | 24.2% event rate for central PE group vs 4.9% for noncentral PE group |
| Tavoly (34) | 2021 | Retrospective cohort | Thrombolytic treatment and/or admission to the ICU | 39.6 | 245 | 42% female | 55+16 | In-hospital | 35.1% event rate for central PE group vs 10.8% for noncentral PE group |
| Ibrahim (35) | 2022 | Retrospective cohort | All-cause mortality | 16.9 | 432 | 47% female | Saddle: 43 (36–58)Non-saddle: 47 (35–62) | In-hospital | 2.7% mortality rate for central PE group vs 3.1% for noncentral PE group |
| Hu (37) | 2023 | Retrospective cohort | Poor prognosis, defined as presence of at least one of the following events: death, cardiopulmonary resuscitation, endotracheal intubation, demand for vasopressors, and reperfusion therapy | 54.6 | 227 | NA | NA | 30-days | 21.0% event rate for central PE group vs 5.8% for noncentral PE group |
| Isath (38) | 2023 | Retrospective cohort | All-cause mortality | 52.9 | 174 | 39% female | 57.4+14.5 | In-hospital | 2.2% mortality rate for central PE group vs 4.9% for noncentral PE group |
| Gleditsch (36) | 2023 | Retrospective cohort | All-cause mortality | 49.9 | 1743 | 48% female | 69 (57–79) | 30-days | 3.8% mortality rate for central PE group vs 4.0% for noncentral PE group |
| Briceño (12) | 2024 | Prospective | All-cause mortality | 1.9 | 848 | 51% female | 72 (59–80) | 30-days | Adjusted all-cause mortality rate was 3.6-fold (95% CI, 1.1–12.4) greater in patients with central PE |
| Hofstetter (39) | 2025 | Retrospective | All-cause mortality | 36.3 | 597 | 45% female | 74 (70–81) | 30-days | 1.8% mortality rate for central PE group vs 3.4% for noncentral PE group |
| Al-Dorzi (40) | 2025 | Retrospective | All-cause mortality | 30.4 | 438 | 64% female | 63 | In-hospital | 5.3% mortality rate for central PE group vs 6.6% for noncentral PE group |
Abbreviations: PE, pulmonary embolism; SD, standard deviation; NA, not available; ICU, intensive care unit.
Five studies had a prospective design [11,12,25,29,41] and 23 studies had a retrospective design [9,13,19–24,26–28,30–40,42]. eTable 2 shows the criteria that were used to define central PE in each of the study cohorts. Demographic features of study populations (age, gender) were similar across the studies, and all the included patients had an objective diagnosis of PE (Table 1). Seven studies only included hemodynamically stable patients [11–13,25,26,30,41]. Twenty-five studies used all-cause mortality as the primary outcome [9,11,12,19–33,35,36,38–42] (eTable 3). Time to study endpoint was different among the studies, varying from the in-hospital stay [20,22,27,28,31,33–35,38,40] up to 30 days.[9,11–13,21,23–26,29,30,32,36,37,39,41,42].
Quality assessment of included studiesRegarding study quality assessment criteria (eTable 4), the study participation was adequate, and the baseline study sample was adequately described in the vast majority of the studies. Nine studies did not adequately describe follow-up [20,22,24,28,30,31,34,35,38]. All the studies provided a clear description of CT-assessed central PE. In only one of the studies an independently-blinded committee adjudicated the etiology of death [12]. Sixteen studies accounted for important potential confounders [11–13,23,24,27,29–34,36,37,39,41]. The majority of the studies used appropriate statistical analyses, which limited the potential for the incorporation of and presentation of invalid results.
OutcomesFor 2 of the studies [23,31], investigators created a control (i.e., patients without central PE) group, and thus the prevalence of central PE could not be calculated. Of the 26 studies with 205,410 participants that enrolled consecutive patients with acute PE, 7748 (3.8%; 95% CI, 3.7–3.9%) had central PE and 197,662 (96.2%; 95% CI, 96.1–96.3%) did not. Overall, 347 of 7528 patients (24 studies providing information on short-term mortality) with central PE died (4.6%; 95% CI, 4.2–5.1%) compared with 6240 of 197,094 without central PE (3.2%; 95% CI, 3.1–3.2%). Pooled results from the 24 studies showed that central PE was associated with 1.2-fold increased odds of short-term all-cause mortality (OR, 1.19; 95% CI, 0.91–1.55; P=0.17; I2=52%) (Fig. 2). The pooled estimate was dominated by the 2 larger studies [28,30], which together provided about 20% of the total patients. There was no evidence of publication bias using the Begg rank correlation method.
Thirteen studies (6428 patients) reported on deaths or complications resulting from PE. Overall, 302 events were observed: 177 in 2027 patients with central PE (8.7%; 95% CI, 7.5–10.1) and 125 in 4401 with noncentral PE (2.8%; 95% CI, 2.4–3.4). Central PE was associated with 2.5-fold increased odds of short-term PE-related deterioration or death (95% CI, 1.44–4.25, P<0.01; I2=70%) (Fig. 3).
Subgroup analysesThe predictive value of central PE with respect to all-cause death was statistically significant when the analysis was limited to 5 studies (2939 patients) that used a prospective design (OR, 1.67; 95% CI, 1.21–2.32; P<0.01), without evidence for heterogeneity (I2=0%) (Table 2 and eFig. 1A). The retrospective (OR, 1.06; 95% CI, 0.76–1.48; P=0.73; I2=69%) studies did not show an association between central PE and all-cause mortality (eFig. 1B). When the studies were categorized by hemodynamic status (only stable patients vs. stable and unstable patients), similar pooled death rates were found among different subgroups (Table 2 and eFig. 2). Pooled results from the 11 studies with 191,956 PE patients that defined central PE as the presence of saddle thrombus (and had available data for short-term all-cause mortality) showed that saddle PE did not have a significant association with the odds of 30-day all-cause mortality (OR, 1.35; 95% CI, 0.75–2.44; I2=71%, P=0.32) (eFig. 3).
Influence of central pulmonary embolism on short-term outcomes: overall and subgroup analyses.
| Variable | No. of articles | No. of participants | No. of central PEs | Odds ratio (95% CI) | I2, % |
|---|---|---|---|---|---|
| Global analysis (random-effects model approach) | |||||
| All-cause mortality | 24 | 204,622 | 7528 | 1.19 (0.91–1.55) | 52 |
| PE-related deterioration or death | 13 | 6428 | 2027 | 2.47 (1.44–4.25) | 70 |
| Subgroup analysesa | |||||
| Design | |||||
| Prospective | 5 | 2939 | 972 | 1.67 (1.21–2.32) | 0 |
| Retrospective | 19 | 201,683 | 6556 | 1.06 (0.76–1.48) | 69 |
| Type of patients | |||||
| Stable | 7 | 9910 | 3702 | 1.42 (1.06–1.92) | 26 |
| Stable and unstable | 17 | 194,712 | 3826 | 1.02 (0.69–1.50) | 68 |
| Type of central PE | |||||
| Saddle PE | 11 | 191,229 | 1976 | 1.35 (0.75–2.44) | 71 |
| Sensitivity analyses (inverse variance fixed-effects meta-analysis) | |||||
| All-cause mortality | 24 | 204,622 | 7528 | 1.21 (1.06–1.39) | 56 |
| PE-related deterioration or death | 13 | 6428 | 2027 | 3.04 (2.35–3.93) | 67 |
Abbreviations: PE, pulmonary embolism; CI, confidence interval.
We reconsidered our findings using inverse variance fixed-effects meta-analysis. Pooled results from the 24 studies showed that central PE was associated with 1.2-fold increased odds of short-term all-cause mortality (OR, 1.21; 95% CI, 1.06–1.39; P<0.01; I2=56%), and with 3.0-fold increased odds of short-term PE-related deterioration or death (OR, 3.04; 95% CI, 2.35–3.93; P<0.001; I2=67%) (eFig. 4).
Pooled results from the 9 studies with 4325 PE patients that had available data showed that central PE had a significant association with the odds of short-term PE-related mortality (OR, 1.77; 95% CI, 1.22–2.55; I2=64%, P<0.01) (Fig. 4A). Four studies (1255 patients) reported on complications resulting from PE. Central PE was associated with 5.3-fold increased odds of short-term PE-related deterioration (95% CI, 3.67–7.68, P<0.001; I2=0%) (Fig. 4B). When we only included studies that assessed in-hospital all-cause mortality [20,22,27,28,31,33,35,38,40], central PE was associated with 1.3-fold increased odds of in-hospital death (OR, 1.30; 95% CI, 0.73–2.34; P=0.38; I2=77%). Five studies reported on PE-related deterioration or death during the in-hospital period [22,27,34,35,40]. Central PE was associated with 2.3-fold increased odds of in-hospital PE-related deterioration or death (95% CI, 1.28–4.28, P=0.01; I2=41%).
DiscussionIn this meta-analysis of 28 studies that included 205,877 patients with acute symptomatic PE, patients with central PE did not have an increased risk of short-term death compared to patients without central PE. In turn, the risk of short-term PE-related complications among patients with central PE was about two times higher than in patients with distal PE. The results were sensitive to nature of data collection (prospective vs. retrospective) and hemodynamic status of patients (only stable vs. stable and unstable). A greater magnitude of risk existed in prospective studies, in hemodynamically stable patients, and for PE-related, rather than all-cause mortality.
In patients with acute symptomatic PE, studies have reported prevalences of central PE that range from 0.8 to 70% [9,42]. However, these studies may be confounded by selection and detection bias. The prevalence of central PE in this meta-analysis (3.8%) confirms that central thrombus are an uncommon finding in unselected patients with PE.
Our analysis showed that central localization of emboli is not associated with a significant increased risk of all-cause death as compared with distal localization. The results are similar to those of a previous meta-analysis which found that central PE was not predictive for all-cause mortality, though it was associated with PE-related outcomes [43]. Alternatively, our findings are partly in contrast with an earlier meta-analysis in which a significant association was found for central embolus location and 30-day mortality after excluding 2 studies that contributed to heterogeneity [44]. This discrepancy may be explained at least in part by the inclusion of a number of more recent studies and more rigorously applied study selection criteria.
Our observations can be explained from a pathophysiologic perspective. Since many patients die of conditions not specifically related to the acute PE event [45], thrombus burden might not have high predictive values for overall mortality. The severity of acute PE event depends on the complex interplay of thrombus location, patient's underlying cardiopulmonary status and compensatory mechanisms [7], and this might explain why central PE had a statistically significant but not clinically strong association with PE-related complications in our study.
What are the implications of these findings? Our results do not support the routine use of central (vs. distal) PE for the identification of low-risk patients for outpatient management since (i) thrombus location was not significantly associated with short-term all-cause mortality, and (ii) clinical prediction rules (combined with the assessment of RV function/size) have demonstrated an excellent accuracy for the identification of low-risk patients with acute PE [46]. Conversely, the localization of emboli is a simple method and is easy to evaluate by visually inspecting the CT images, and assessment of central embolus location might be combined with other predictors (e.g., vital signs, cardiac biomarkers, predictors of RV stress) to develop scores aiming at identifying PE-related mortality and PE-related decompensation [10]. Future studies should determine whether models incorporating thrombus location might help clinicians weigh the risks and benefits of reperfusion therapies and will allow for enrichment of prospective studies and trials designed to treat PE.
This meta-analysis has limitations. Substantial heterogeneity was observed and contributed to lowering the evidence. Sensitivity analyses were attempted through subgroup analyses, which showed reduced heterogeneity for prospective studies, studies that enrolled hemodynamically stable patients with PE, and studies that reported PE-related complications. Because adjudication of death events is complex [47], the study design and hemodynamic status are likely factors in the variability of estimates. More research in specific areas is needed to fully assess how these factors play a role in heterogeneity. The choice of all-cause mortality as the primary outcome could be questionable. However, while PE-specific mortality might better reflect prognosis associated with thrombus location, all-cause mortality captures the broader impact of PE on a patient's overall health and well-being, including complications and other health conditions. Limitations of each of the included studies may have introduced significant biases into this meta-analysis's estimates of the prognostic value of central PE. The definition of central PE varied across studies, which might affect the generalizability of the findings. Our study was based on study-level meta-analysis of individual observational studies, and we did not have sufficient information that would allow us to adjust for confounding variables. Meta-analyses of individual patient data could have potentially allowed for such adjustments. Finally, this meta-analysis included one study with no events in either study group [22]. While some researchers have suggested the exclusion of these studies from meta-analyses, this approach may introduce systematic bias by inflating the estimated event rates. Of note, when we repeated analyses after excluding the study by Ryu et al. [22], the findings were largely similar to our original analyses.
In conclusion, this systematic review showed that central PE did not have an association with increased risk of all-cause mortality, while they were significantly associated with an increased risk of PE-related complications, including PE-related death, within 30 days of diagnosis.
Authors’ contributionsConcept and design: Briceño, Velasco, Jiménez.
Acquisition, analysis, or interpretation of data; statistical analysis: Briceño, Velasco, Castillo, Jara, Lago, Yong, Díaz, Piazza, Bikdeli, Jiménez.
Drafting of the manuscript: Briceño, Velasco, Piazza, Bikdeli, Jiménez.
Study supervision: Bikdeli, Jiménez.
Dr. Jiménez had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Artificial intelligenceAuthors did not use artificial intelligence tools during manuscript writing.
Funding sourcesNone.
Conflict of interestWB has nothing to disclose.
JMV has nothing to disclose.
AC has nothing to disclose.
IJ has nothing to disclose.
LL has nothing to disclose.
EY has nothing to disclose.
GD has nothing to disclose.
G.P. reports research grants paid to his institution from BMS/Pfizer, Janssen, Alexion, Bayer, Amgen, BSC, Regeneron, and NIH 1R01HL164717-01 and consulting fees from BSC, Amgen, NAMSA, BMS/Pfizer, and Janssen.
B.B. was supported by a Career Development Award from the American Heart Association and VIVA Physicians (#938814). Dr. Bikdeli was supported by the Scott Schoen and Nancy Adams IGNITE Award and is supported by the Mary Ann Tynan Research Scientist award from the Mary Horrigan Connors Center for Women's Health and Gender Biology at Brigham and Women's Hospital. Dr. Bikdeli reports that he was a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. Dr. Bikdeli has not been involved in the litigation in 2022–2025 nor has he received any compensation in 2022–2025. Dr. Bikdeli reports that he is a member of the Medical Advisory Board for the North American Thrombosis Forum (VasuLearn Network), and serves in the Data Safety and Monitory Board of the NAIL-IT trial funded by the National Heart, Lung, and Blood Institute, and Translational Sciences. Dr. Bikdeli is a collaborating consultant with the International Consulting Associates and the US Food and Drug Administration in a study to generate knowledge about utilization, predictors, retrieval, and safety of IVC filters. Dr. Bikdeli receives compensation as an Associate Editor for the New England Journal of Medicine Journal Watch Cardiology, as an Associate Editor for Thrombosis Research, and as an Executive Associate Editor for JACC, and is a Section Editor for Thrombosis and Haemostasis (no compensation).
DJ has nothing to disclose.
Briceño and Jiménez authored studies assessed by this meta-analysis.
We would like to acknowledge RV Hofstteter and D Aujesky (Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland), and M Monreal (Faculty of Health Sciences, UCAM-Universidad Católica San Antonio de Murcia, Murcia, Spain) for providing unpublished data that were included in this analysis.