Pneumonia continues to rank among the leading causes of mortality globally, representing a substantial health burden due to its clinical heterogeneity and severity. Despite significant biomedical progress over recent decades, therapeutic strategies have remained largely unchanged. Management still relies primarily on empirical antibiotic therapy and supportive measures, with little advancement in precision-guided interventions [1]. The rapid and exhaustive research efforts during the COVID-19 pandemic have demonstrated that immunomodulatory treatment for pneumonia is feasible, exemplified by the introduction of anti-interleukin 6 therapy. This paradigm shift must now extend to non-COVID-19 community-acquired pneumonia (CAP), thereby necessitating a deeper understanding of its pathophysiology.
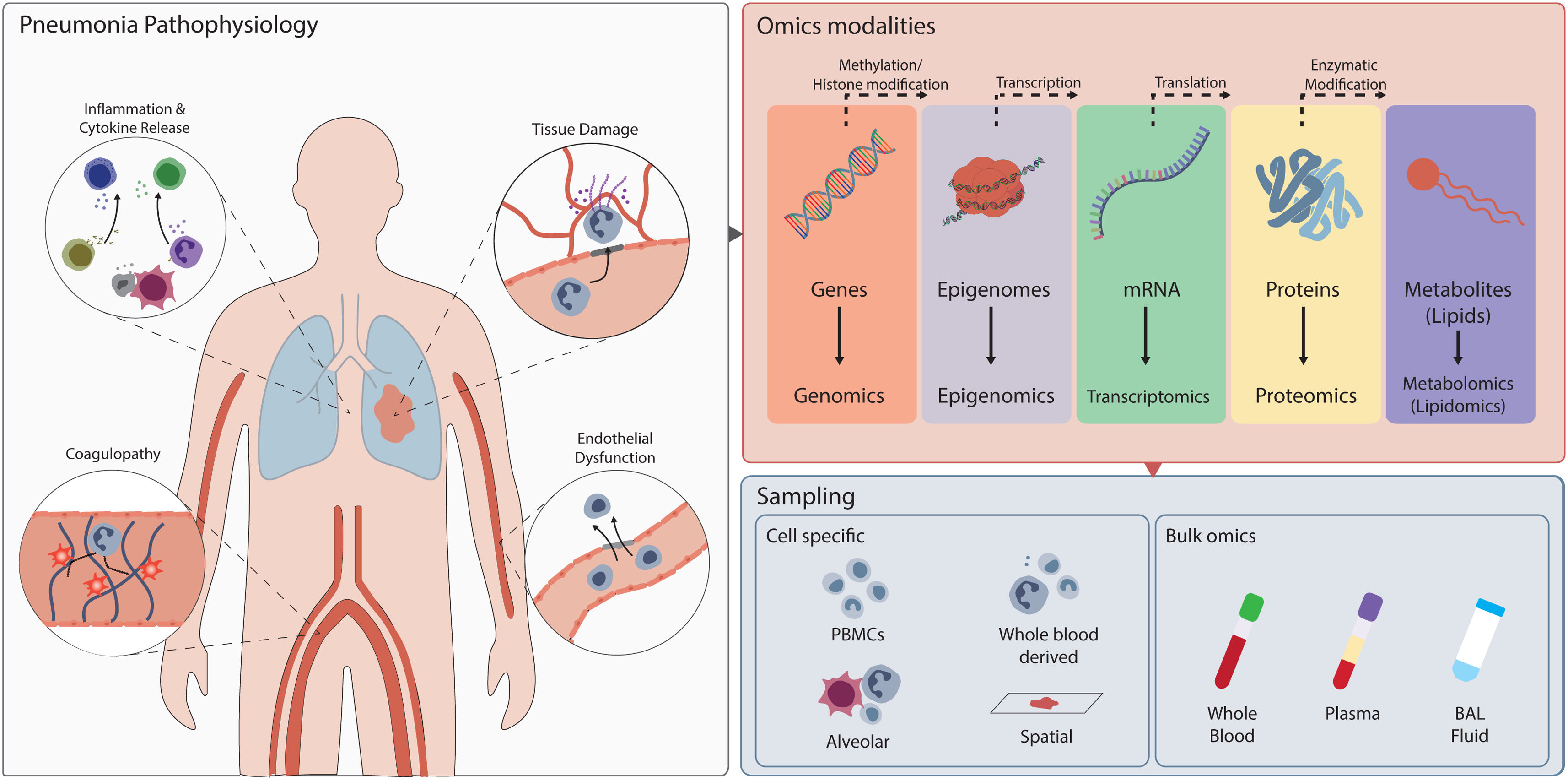
Although pneumonia is typically viewed as a localized pulmonary infection, it is a systemic disease with broad pathophysiological consequences (Fig. 1) [2]. The initial insult in the alveolar compartment triggers a cascade of immune and inflammatory responses that can spill over into the systemic circulation. Among the key systemic manifestations is coagulopathy, which arises from endothelial injury and cytokine dysregulation. Concurrently, damage-associated molecular patterns released from injured cells together with bacterial products amplify the inflammatory cascade, leading to vascular leakage and tissue hypoxia, ultimately culminating in multi-organ failure [2]. This complex interplay of pathophysiological processes, collectively termed the “dysregulated host response”, varies significantly between individuals. Some of these processes can be assessed using routine laboratory measurements, but in recent decades, the rise of “omics” technologies has enabled much more detailed studies of the host response.
Overview of the pathophysiological mechanisms involved in pneumonia and the types of omics technologies used to study them. Omics approaches spanning genomics to metabolomics enable mapping of these responses across biological layers. Immune cells can be isolated from peripheral blood mononuclear cells (PBMCs), whole blood, or the alveolar niche, allowing either parallel analysis of multiple subsets to study their coordinated activity, or isolation of specific cell types such as neutrophils for dedicated omics profiling.
Omics technologies allow us to dive deeply into cell biology and study the body at the molecular level. Genomics, epigenomics and transcriptomics enable us to study genes, their epigenomic state and their transcripts, while proteomics detects the proteins encoded by these transcripts. The scope of research has recently expanded to include cellular metabolism, as metabolic disturbances are increasingly recognized to contribute to immune dysregulation. Initially, these technologies were applied to whole blood or plasma, often referred to as “bulk” omics. However, with the increased availability of new technologies like single-cell RNA sequencing, several of these methods can now be employed at a single-cell resolution [3]. Furthermore, the rapidly growing field of spatial omics enables the study of tissues on a slide at a similar detailed level, allowing us to understand cellular function within its environmental context [4].
Omics technologies have revealed substantial heterogeneity within CAP, demonstrating that patients with seemingly similar clinical symptoms can exhibit remarkably divergent biological responses at the molecular level. For example, bulk transcriptomic analyses of whole blood samples from patients with severe CAP have been instrumental in identifying distinct host response signatures [5]. These signatures can stratify patients into different immunological states, some displaying profiles indicative of immunosuppression, characterized by features such as T-cell exhaustion and reduced Human Leucocyte Antigen-DR expression, while others exhibit heightened immune activation. Bulk proteomics analyses have provided additional insight into host response changes in patients with moderately severe CAP. One investigation quantified 3482 proteins in neutrophil lysates, which were functionally linked to increased (aerobic) metabolism and (messenger) RNA translation/processing, and decreased cytoskeletal organization, changes that correlated with a longer time to clinical stability [6]. Another study detected 2676 proteins in plasma; proteins that were more abundant in patients than in controls (one third) were associated with innate immune and mitosis pathways, and mainly originated from lung and cardiac tissue [7]. Lipidomic analyses of blood monocytes and neutrophils from CAP patients showed marked alterations, affecting lipid species, classes, and saturation [8]. Lipids were generally decreased in monocytes while increased in neutrophils. Integration of lipidomic and transcriptomic data revealed disrupted sphingolipid metabolism in both cell types, which was associated with reduced cytokine responses upon stimulation with bacterial lipopolysaccharide, thereby linking lipid modifications with immune function. Plasma lipidomics conducted in patients with severe CAP showed overall decreased lipid levels shortly after admission to the intensive care unit, with a subsequent recovery during follow up [9]. Interestingly, the rise of certain lipids, such as cholesterol esters, tended to correlate with poorer patient outcomes.
More recently, researchers have gained valuable insights by directly examining the site of infection through single-cell transcriptomics performed on bronchoalveolar lavage fluid [10]. These studies showed that neutrophils and macrophages largely drive inflammation within the lung, primarily mediated by the release of S100/A8/A9 and interleukin 8, which are strongly implicated in the pathology of severe CAP. Conversely, an increase in humoral and T-cell mediated immune responses was found to be associated with a milder disease course.
The future of CAP management hinges on a more complete understanding of its pathophysiology, achievable only through the integration of diverse omics techniques. The critical challenge lies in moving beyond the current landscape of disparate phenotypes identified through single-layered, descriptive analyses towards constructing an integrated understanding of CAP. This necessitates designing studies that employ multi-omics technologies in the same patient, such as genomics, transcriptomics, proteomics, and metabolomics, in concert. By aligning these layers of biological information, researchers can begin to trace molecular cascades from genetic variation through transcriptomic regulation and protein translation to metabolic alterations. Such integrative study designs will provide a comprehensive view of CAP pathogenesis, linking host response mechanisms to clinical outcomes, while also revealing patient heterogeneity and potential therapeutic targets, crucial steps towards personalized medicine.
Implementing omics-driven insights requires rapid, bedside biomarker measurement. While comprehensive omics analyses are powerful for research, their complexity and costs limit acute clinical utility. Comprehensive “omics” data can be distilled into limited sets of biomarkers and emerging point-of-care diagnostics can offer quick and efficient assessment of such biomarkers. As an example, Inflammatix's Triverity system provides insights into the host response by measuring the expression levels of selected RNAs, identified through bulk transcriptomic analyses of large patient cohorts, providing insight into the likelihood of the pathogen type (bacterial versus viral) and an adverse clinical outcome [11]. Similarly, benchtop systems such as the Randox's MultiSTAT or the Ichroma by Boditech offer the capability to rapidly quantify protein levels. Even portable flowcytometers are currently under development, enabling single-cell analysis at the point of care [12]. Omics approaches are essential in selecting the most informative analytes for such platforms, tailoring diagnostics to reflect the patient's host response. Notably, these approaches have not yet been implemented in CAP. One of the central debates in the management of severe CAP concerns the use of corticosteroids. Major randomized controlled trials have yielded conflicting results: REMAP-CAP was halted early due to a lack of mortality benefit, whereas CAPE-COD demonstrated a survival advantage [13,14]. While such discrepancies may partly reflect differences in trial design and patient selection, they also highlight the substantial heterogeneity in host responses among CAP patients. Recent evidence indicates that corticosteroid therapy may confer the greatest benefit in individuals with elevated C-reactive protein levels, suggesting that the inflammatory burden may modulate treatment efficacy [15]. These insights underscore the potential of advanced biomarker-based stratification, moving beyond C-reactive protein alone, to guide immunomodulatory treatment decisions more precisely.
In conclusion, advancing towards personalized medicine in pneumonia is not only necessary but increasingly feasible, with omics technologies providing a critical foundation. These approaches enable precise characterization of individual patient profiles and, when combined with rapid point-of-care biomarker assessments, hold the potential to fundamentally reshape clinical management. By moving beyond generalized treatment paradigms to targeted, data-driven interventions, we can improve outcomes and shift pneumonia care towards a more proactive and precision-guided approach.
FundingS.C.M. Joosten is supported by a grant from Eurostars (SEPRAID, Project E!30).
Conflicts of interestNone declared.