Tuberculosis (TB) remains a major global health burden, causing more than 10 million new cases and 1.6 million deaths each year. Currently, the only approved TB vaccine in use in humans, is the one hundred years old vaccine, BCG, an attenuated vaccine derived from an isolate of Mycobacterium bovis that causes TB in cattle. BCG shows a variable efficacy in preventing pulmonary forms of the disease in humans, so new vaccines are needed to help stop TB transmission. Among the 15 diverse TB vaccine candidates in clinical trials, MTBVAC is the only one based on rational attenuation of a human clinical isolate of Mycobacterium tuberculosis, which contains the largest number of antigens of the TB vaccine candidates in the pipeline. MTBVAC was designed and constructed as a response to the need to confer a better TB protection in terms of pulmonary disease prevention in newborns, adolescents, and adults. This review aims to provide an overview of the preclinical and clinical development of MTBVAC to the present. We will focus on the clinical development of MTBVAC, and we will compare it with other TB vaccine candidates currently in Phase 3 efficacy trials.
Tuberculosis (TB) is an infectious disease that ranks among the leading causes of death worldwide. Its causative agent is Mycobacterium tuberculosis, also known as the Koch bacillus, discovered by Robert Koch in 1882.1 Scientific community has extensively studied TB, revealing the early-stage relationship between the development of the disease and the immune status of patients.2
A significant part of the bacillus’ infectivity is due to its person-to-person transmission through the respiratory route. Transmitted through aerosols, these deposit in the pulmonary alveoli, where they infect alveolar macrophages.3 The lung is the primary target organ of M. tuberculosis, although 10–20% of immunocompetent patients develop extrapulmonary forms of the disease, a figure that increases to 60% in immunocompromised patients.4
Before the arrival of the pandemic caused by the SARS-CoV-2 coronavirus (COVID-19), TB was the leading cause of death caused by a single infectious pathogen, surpassing HIV/AIDS, and malaria. It is estimated that a quarter of the global population (more than two billion people worldwide) is infected with M. tuberculosis, although only 5–10% will develop the disease throughout their life.5 Furthermore, due to its high prevalence in developing countries, transmission appears to be related to migratory movements, as well as contexts of poverty and overcrowding.6,7
One of the most pressing aspects is the increasing resistance cases of TB, reflected in the emergence of multidrug-resistant (MDR-TB) TB that does not respond to at least isoniazid and rifampicin, the 2 most powerful anti-TB drugs and extensively drug-resistant (XDR-TB) MDR TB that is resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin) M. tuberculosis strains. This has led to the need to find new ways to combat the disease with better diagnosis of TB, increased discovery of new drugs, shortening treatment time and decreasing transmission, particularly through preventive measures.8,9
Currently, vaccination is considered the most cost-effective measure in preventing a disease and the development of resistance to its treatment.9 The only licensed vaccine for TB prevention is Bacillus Calmette-Guérin (BCG) vaccine, which is based on the attenuation of Mycobacterium bovis originally isolated from cattle.10 First administered over a century ago, the World Health Organisation (WHO) recommends its use in countries with incidence higher than 10 TB cases per 100,000 population per year.11 BCG provides protection with an efficacy rate of 60–80% against meningeal and miliary TB, which are disseminated and aggressive forms of the disease that occur during childhood.12 However, clinical trials have shown variable efficacy preventing pulmonary forms, the transmissible form of the disease, especially in adolescents and adults.9,13 BCG has been shown to provide protection against TB for up to 10–15 years post-vaccination.14 Several hypotheses have been proposed to explain this variation in efficacy, including the variability between different BCG strains, dosage, route of administration,15 patient's genetic profile, nutritional status,16,17 viral or helminthic infections,16 and exposure to environmental mycobacteria17,18 or geographical variation.12
During the subcultivation process of BCG attenuation between 1908 and 1921, more than 100 additional genes were lost from BCG relative to M. tuberculosis. Among them, deletion of Region of Difference 1 (RD1). Many authors attribute the limited efficacy of BCG to its antigenic composition, insufficiently related to that of M. tuberculosis. BCG lacks 23–28% of the CD4 epitopes present in the pathogen,15 including ESAT6 and CFP10 coded in RD1, which is present in M. tuberculosis and absent in BCG, being mainly responsible for the attenuation of BCG.19
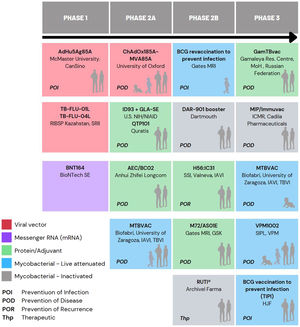
Currently, there are 15 different candidates in clinical trials in the global TB vaccine pipeline, which are included in Fig. 1 of this article.5,20
Studies on subunit vaccine candidate M72/AS01E have shown promising results, demonstrating its ability to reduce the risk of developing active disease in individuals previously infected with M. tuberculosis, achieving disease prevention (POD) in approximately 50% of the volunteers studied.21
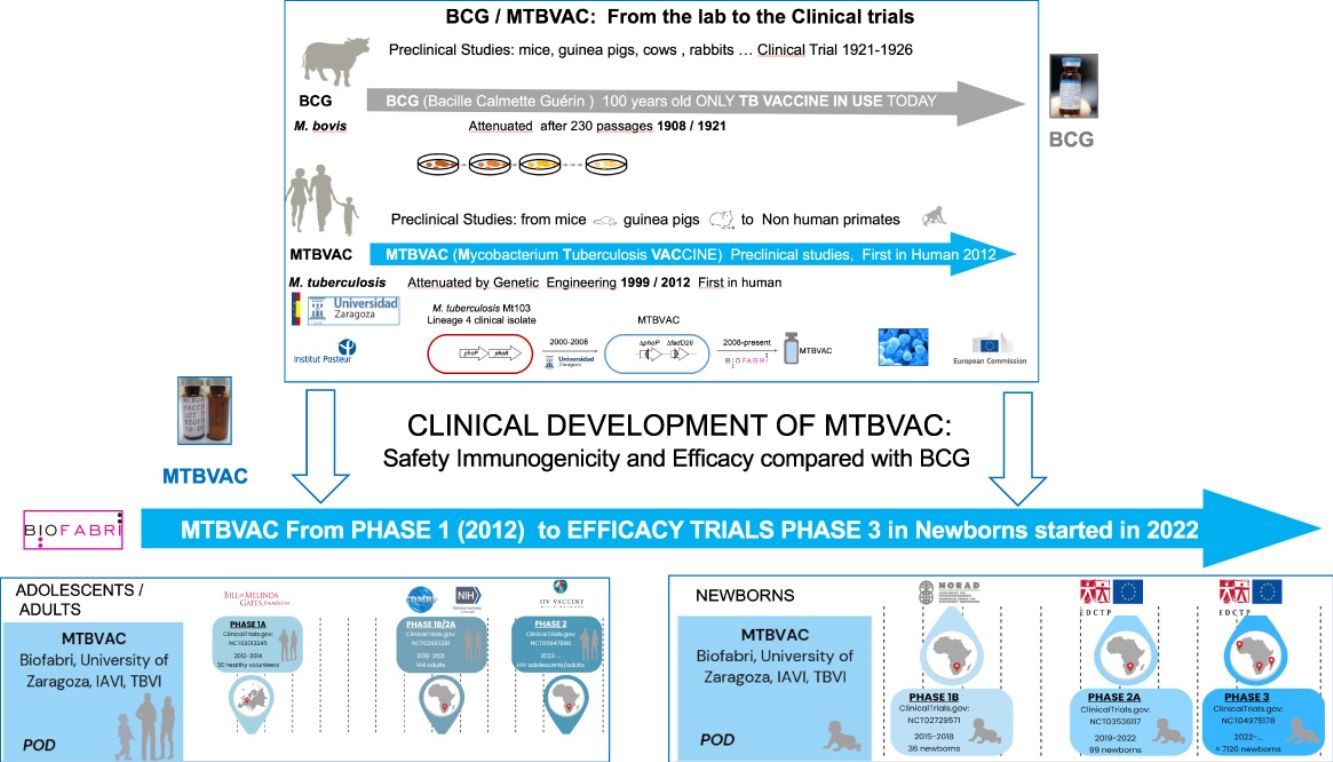
Therefore, the need for new vaccines to improve BCG protection led to the development of several candidates, including (Mycobacterium Tuberculosis Vaccine (MTBVAC), based on the rational attenuation of M. tuberculosis. Developed by the University of Zaragoza (Spain) in collaboration with the Pasteur Institute (France), MTBVAC has demonstrated the ability to induce a comprehensive, pathogen-specific immune response superior to the current BCG vaccine in different animal models (Martín et al., 2021). Since 2008, the Spanish biopharmaceutical company Biofabri has been the industrial partner of the University of Zaragoza and responsible for the clinical and industrial development of the vaccine in collaboration with TuBerculosis Vaccine Initiative (TBVI) and US International AIDDS Vaccine Initiative (IAVI). Currently, MTBVAC is progressing with its Phase 3 efficacy trial in newborns in Sub-Saharan Africa.9
MethodologyA scoping review of international publications over the last 20 years was conducted to cover the complete development of MTBVAC. A literature search was performed in the PubMed database using the key terms “Tuberculosis”, “Prevention and control”, “Vaccines”, “Attenuated vaccines” and “MTBVAC”. After selecting the articles and removing duplicates, a first screening phase was conducted by reading the abstract, followed by a second phase of reading the full text to exclude irrelevant articles. During the review process, a couple of articles were added based on citation identification.
Additionally, multiple searches were conducted in the ClinicalTrials.gov database to determine the status of clinical trials associated with each referenced vaccine.
Mycobacterium tuberculosis vaccine: MTBVACMTBVAC has been designed and constructed with the aim of providing better protection than the current BCG against respiratory forms of the disease. In its conception we have considered the experience of 100 years of BCG use, both being live attenuated vaccines, can be administered intradermally in a single dose and the same distribution and storage methods can be used. MTBVAC aims to be a universally accessible and affordable vaccine for countries with the highest TB burden that protects better than BCG against pulmonary TB. MTBVAC is postulated as a preventive vaccine with better protection than BCG at birth, and as a revaccination strategy in adolescents and adults previously vaccinated with BCG in TB-endemic countries.22
MTBVAC was constructed by rationally attenuating a clinical isolate of M. tuberculosis Mt103, belonging to Lineage 4 (Euro-American), one of the most globally widespread modern lineages of M. tuberculosis.23 According to the first Geneva consensus published in 2005,24 it was designed based on the construction of a strain of M. tuberculosis with two stable and independent genetic deletions without antibiotic resistance markers, located in the phoP and fadD26 genes, conferring a PhoP-/PDIM-phenotype to MTBVAC, and maintaining the rest of the antigens present in the bacillus and absent in the current BCG vaccine.25 Today MTBVAC is the only vaccine based on the attenuation of M. tuberculosis itself.26,27
PhoPR is a two-component signal transduction system that is highly relevant to the pathogenesis of M. tuberculosis. PhoP acts as a transcriptional regulator of a wide variety of genes related to functions involved in the pathogenicity of the bacillus, controlling up to 2% of its genome.28,29 PhoPR was identified as a major factor of virulence in TB while studying an MDR-TB outbreak caused by a M. bovis strain that showed an increased expression of phoP gene due to an insertion sequence IS6110. This MDR-TB outbreak resulted in more than a hundred deaths in Spain during the 1990s, primarily among AIDS patients before triple therapy against HIV was implemented.26
PhoPR controls the biosynthesis of several lipid components of the cell wall, such as diacyltrehalose (DAT), polyacyltrehalose (PAT) and sulfolipids (SL), responsible for inhibiting the host's innate response and inducing a productive cough that facilitates the transmission of the pathogen.30 In turn, it regulates the expression of ESX-1, responsible for the secretion of the virulence factor ESAT6,29 which when secreted inhibits autophagy, a mechanism responsible for the destruction of the intracellular pathogens, and induces apoptosis of infected cells, facilitating the spread of the bacillus between cells.31 Finally, PhoPR acts on a small RNA strand (mcr7), which represses the translation of tatC, a protein belonging to the TAT (Twin Arginine Translocation) system, responsible for antigen secretion, thus reducing the secretion of Ag85A and Ag85C. MTBVAC, as a phoP deletion mutant, shows the absence of cell wall components such as DAT, PAT, and SL. Furthermore, phoP deletion inhibits ESAT6 secretion and increased secretion of Ag85 major tuberculous antigens30,31 and increases production and secretion of the second messenger c-di-AMP, which could be relevant for enhancing the innate immune response of the host.30
The fadD26 gene is responsible for the biosynthesis of lipid components of the M. tuberculosis cell wall called phthiocerol dimycocerosates (PDIMs) a major virulence factor in M. tuberculosis strains. PDIMs function lies in the disruption of the phagosome, in conjunction with the action of ESAT6, making them crucial for the survival of the pathogen within the host. The absence of PDIMs because of the deletion of fadD26 in MTBVAC increases the susceptibility of the pathogen to the innate immune response, as it is unable to prevent the maturation of the phagosome, which leads to death of the bacterium.29,31
Preclinical studies on prototype vaccine SO2 and MTBVACThe phoP gene was inactivated in a M. tuberculosis clinical isolate by insertion of a kanamycin resistance marker into it. The resulting strain, called SO2, also contained a single mutation in the PDIM locus, conferring it a PhoP- and PDIM-deficient double phenotype. SO2 was used as a prototype TB vaccine candidate (PDIM-deficient phoP mutant) in preclinical animal models to study attenuation and protection against TB challenge.25,32,33 SO2 preclinical studies confirmed a superior attenuation profile to BCG,34 as well as better protection in guinea pigs35 (Table 1). SO2 did not meet the Geneva Consensus criteria required to enter clinical trials, due to the inactivation of PhoP through the insertion of an antibiotic resistance marker and a single mutation in the fadD26 gene.
Preclinical studies conducted with the SO2 vaccine prototype.
| Author (year) | Study | Aim | Results |
|---|---|---|---|
| Pérez E et al. (2001)32 | Evaluation of the role of phoP gene in the virulence of M. tuberculosis | To analyse how the presence and activity of the phoP gene affects the ability of the bacterium to cause disease in the host | Mutations in the phoP gene significantly weakened the bacterium's ability to survive and proliferate in the host, suggesting that this gene is crucial for the bacteria's adaptation and persistence in the human body |
| Williams A et al. (2005)34 | Evaluation of vaccines in the EU TB vaccine cluster project in a guinea pig aerosol infection model of TB | To evaluate the protection conferred by TB vaccine candidates | 24 TB vaccines candidates were studied. Several promising candidates were identified including MVA Ag85 boosting BCG and SO2 |
| Martin C et al. (2006)35 | Evaluation of protective immunity of SO2 against TB in mice and guinea pigs | To study safety and reactogenicity of SO2 compared with parent strain M. tuberculosis Mt103 and BCG. | SO2 proves to be more attenuated than M. tuberculosis Mt103 and BCG And higher CD4+ and CD8+ immunogenicity |
| Cardona PJ et al. (2009)33 | Extended safety studies of SO2 in mice and guinea pigs | To study safety profile, stability and lack of toxicity, both in a pre- and post-exposure model in mice and Guinea pigs. | SO2 showed absence of toxicity and stability of the phoP mutation |
| Verreck FAW et al. (2009)36 | Evaluation of efficacy against TB in Rhesus Macaques | To evaluate immunogenicity and protection against M. tuberculosis in rhesus macaques | MVA.85A Boosting BCG and SO2 show protection in non-human Primates |
MTBVAC was constructed by two independent stable deletions in phoP and fadD26 genes allowing, thereby enabling its progression into clinical development.25,27 Since then, MTBVAC has demonstrated safety, protection, and immunogenicity comparable to prototype SO2 in studies in mice, guinea pigs25 and rhesus macaques.36,37 In addition, MTBVAC confers superior protection to BCG in adult and neonatal mouse models38 and guinea pigs22 (Table 2).
Preclinical studies conducted with the MTBVAC vaccine.
| Author (year) | Study | Aim | Results |
|---|---|---|---|
| Arbues A et al. (2013)25 | Construction, characterisation, and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials | To describe the construction of MTBVAC from the SO2 prototype and preclinical evaluation following Geneva consensus: two independent deletions and non-antibiotic markers. | MTBVAC is functionally and phenotypically comparable to SO2 in several animal models studies prior to its entry into human clinical trials. |
| Aguilo N et al. (2016)38 | Study of safety, immunogenicity, and protective efficacy of MTBVAC against M. tuberculosis in newborn mice. | To assess the safety and immunogenicity of MTBVAC in newborn mice to support Phase 1b trials in human neonates. | MTBVAC is safe, immunogenic and protects against M. tuberculosis and does not affect developing organs in newborn mice. |
| Clark S et al. (2017)22 | Study of revaccination BCG and MTBVAC in Guinea Pigs. | To study MTBVAC and BCG, administered individually or in combination. | MTBVAC improves BCG's protection against M. tuberculosis supporting strategy to boost the effects of BCG vaccinated at birth in adolescents and adults in TB-endemic countries. |
| Díaz C et al. (2019)29 | Comparative Metabolomics between M. tuberculosis and the MTBVAC | To compare the differences between metabolites produced by MTBVAC and M. tuberculosis. | 34 metabolites were identified, of which 25 were increased in MTBVAC and 9 in M. tuberculosis. |
| Pérez I et al. (2020)23 | Study MTBVAC representing the three modern M. tuberculosis lineages reveal that the Euro-American genetic background. | To evaluate the efficacy of the new variants of MTBVAC (L2 and L3 strains) compared to the original (L4 strain) and determine whether MTBVAC can protect against three modern strains. | All three variants of MTBVAC confer protection against the disease irrespective of the phylogenetic lineage used as the basis for their construction. Greater protection is observed for the variant based on the L4 lineage. |
| White AD et al. (2021)37 | Protection study of intradermal vaccination with MTBVAC in rhesus macaques against aerosol challenge with M. tuberculosis | To determine the protection of a single intradermal dose of MTBVAC in rhesus macaques and to characterise the immune response for comparison with that defined in both preclinical studies in macaques and in human clinical trials. | MTBVAC vaccination conferred significantly improved protection against the BCG vaccinated group and the non-vaccinated group. Immunological profiles demonstrated a predominantly Th1-type response that correlated with results in clinical and preclinical trials. |
| Pérez I et al. (2022)30 | Metabolomic study of MTBVAC | To analyse the expression of the second messenger c-di-AMP in MTBVAC and its impact on vaccine efficacy. | Deletion of phoP in MTBVAC induces increased production of c-di-AMP, which influences attenuation and efficacy, leading to an increased IL-1β-mediated immune response to BCG. |
In 2012, MTBVAC vaccine candidate was approved for entry into human clinical trials.
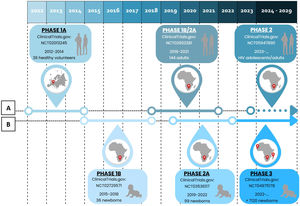
MTBVAC clinical trialsPHASE 1a. Dose-escalation study to assess the safety and immunogenicity of MTBVAC vaccine compared to BCG vaccineBetween 2012 and 2014, a randomised, double-blind, BCG-controlled clinical trial was conducted in Lausanne (Switzerland) to assess the safety and local tolerability of MTBVAC in healthy adults aged 18–45 years.39 Thirty-six (36) participants were randomised into three cohorts, verifying its safety by progressively increasing the MTBVAC dose [low-dose (5×103CFU); intermediate-dose (5×104CFU); and high-dose (5×105CFU)], in a 3:1 ratio. As primary objective of the study, MTBVAC showed a safety profile comparable to BCG, with no serious adverse effects. MTBAVC was shown to be at least as immunogenic as BCG with evidence of dose-dependent induction of specific CD4+ T cells (IFN-γ+, TNF-α+, IL-2+) in both groups, although the study sample did not allow for statistically significant differences. The results obtained suggested that both BCG and MTBVAC predisposed to the development of a central memory T-cell-mediated immune response, rather than an effector memory T-cell-mediated response, the former being essential for the maintenance of long-term immunological memory with a greater tendency in MTBVAC40 (Fig. 2).
Clinical development of MTBVAC. (A) Clinical development in adolescents and adults. (B) Clinical development in newborns. Phase 1a (2012–2014). Phase 1b (2015–2018). Phase 1b/2a (2018–2021). Phase 2a (2019–2022). Phase 3 (2022). Phase 2 (2023).
Between 2015 and 2018, a new trial was conducted in Worcester, near Cape Town (South Africa) with the aim of evaluating the safety and immunogenicity of MTBVAC in newborns in a TB endemic area.41 Prior to this, a safety arm of 18 adult participants was developed in which participants were assigned to the MTBVAC and BCG groups in a 1:1 ratio. Once the safety results of this cohort were obtained, the study was conducted by distributing 36 newborns in a 3:1 ratio in three cohorts of progressive doses of MTBVAC [low-dose (2.5×103CFU); intermediate-dose (2.5×104CFU); and high-dose (2.5×105CFU)].
The study showed that MTBVAC had an acceptable reactogenicity profile, inducing as in the phase 1a trial, a dose-dependent Th1 response mediated by polyfunctional CD4+ T cell. Thus, significantly reduced responses were observed in the low-dose group (2.5×103CFU) and therefore this dose was discontinued in future studies. On the other hand, statistically significant responses were observed between the high-dose group (2.5×105CFU) and the BCG group. In addition, reduced frequencies of CD4+ Th17, Th22 and CD8+ T lymphocytes were observed. High IFN-γ conversion frequencies were observed for the first time in the IGRA test, due to cross-reactivity with CFP10 antigens42 that correspond with the protection in animal models43 (Fig. 2).
PHASE 1b/2a. MTBVAC study in adults with and without latent TB infection in South-Africa (A-050)In 2018, MTBVAC initiated a Phase 1a/2b trial in South Africa with the aim of defining the appropriate dose of inoculation, as well as evaluating the safety and immunogenicity of MTBVAC in adults with or without latent TB disease previously vaccinated with BCG in childhood. For this purpose, 144 participants were selected and distributed into 8 cohorts 1–4 those without TB disease and 5–8 those with TB disease. Progressive doses of MTBVAC were used: 5×103CFU in cohorts 1 and 5, 5×104CFU in cohorts 2 and 6, 5×105CFU in cohorts 3 and 7, 5×106CFU in cohorts 4 and 8. The study has been completed and the results are pending publication44 (Fig. 2).
PHASE 2a. Safety and immunogenicity study to define the dose of MTBVAC in South African neonatesFinally, a concurrent Phase 2a trial was initiated in South Africa in 2019 to define the appropriate dose of administration in newborns, evaluate the safety and immunogenicity of MTBVAC, as well as to assess the frequency of IGRA test conversion, following the results of the Phase 1b trial. 99 HIV-unexposed infants without BCG vaccination were randomised into three cohorts in a 3:1 ratio [25 participants were assigned to each MTBVAC progressive dose group (2.5×104CFU, 2.5×105CFU and 2.5×106CFU) and 24 to the BCG group]. The study has been completed and the results are pending publication45 (Fig. 2).
PHASE 3. Efficacy, safety and immunogenicity evaluation of MTBVAC in newborns in sub-Saharan AfricaCurrently, as of September 2022, MTBVAC received partial funding through the European & Developing Countries Clinical Trials Partnership (EDCTP2) to conduct a multi-centre Phase 3 efficacy trial in newborn babies. This is a randomised, double-blind, BGG-controlled, multi-centre clinical trial, with the evaluation being conducted in six different sites, four of them in South Africa for efficacy study, one in Senegal and one in Madagascar for safety and immunogenicity compared to BCG. The objective of this study is to evaluate the safety, immunogenicity and, most importantly, the efficacy of MTBVAC in healthy HIV-negative infants born to HIV-positive or HIV-negative mothers. A total of 7120 participants are expected to be recruited and randomised 1:1 to either the MTBVAC or BCG group. Recruitment is currently underway, and the trial is expected to end in September 202946 (Fig. 2).
Comparison of MTBVAC with other candidate vaccines in PHASE 3In addition to MTBVAC, other vaccine candidates are in Phase 3 of clinical development, such as VPM1002, GamTBvac, Immuvac (MIP), and revaccination with BCG5 (Fig. 1).
VPM1002 is a live attenuated vaccine based on recombinant BCG that has demonstrated safety and immunogenicity in Phase 1 and 2a clinical trials. Its potential application as a post-exposure immunisation technique in adults and as a preventive method for TB in newborns is being studied.47 It was designed to stimulate the CD8+ T cell-mediated response, resulting in an increase in IL-17 production.48
GamTBvac is a subunit-based vaccine containing ESAT6-CFP10 antigens. GamTBvac has shown an acceptable safety profile while inducing a specific and durable Th1 and humoral immune response.49 This specific Th1 cellular response is mediated by IFN-γ, with a better profile at medium doses, showing a progressive increase in IFN-γ production without a significant subsequent decline in cytokine production over time.50
The main difference between MTBVAC and the other candidate TB vaccines in Phase 3 is its larger antigen composition. MTBVAC is the first and only vaccine candidate based on the attenuation of M. tuberculosis in clinical trials.31 BCG was developed over a century ago through repeated subculture attenuation of M. bovis.51 On the other hand, VPM1002 is a recombinant BCG vaccine (BCGΔureC::hly) in which the urease C gene has been replaced by the coding gene (hly) of listeriolysin O from Listeria monocytogenes.47 Immuvac (MIP) is a vaccine based on heat-killed Mycobacterium indicus pranii.9 Finally, GamTBvac is a recombinant subunit vaccine formed by the fusion of two mycobacterial antigens (Ag85A and ESAT6-CFP10) with the dextran-binding domain immobilised on dextran, mixed with an adjuvant core consisting of DEAE-dextran with CpG oligodeoxynucleotides (TLR9 antagonists).52
Currently, the most reliable criterion for efficacy is the demonstration of disease prevention. Therefore, all the mentioned vaccines are conducting trials to demonstrate their efficacy in this regard.46,53–55 Additionally, revaccination with BCG is conducting a study to demonstrate prevention of M. tuberculosis infection, which is its primary indication.56 Furthermore, VPM1002 is studying the prevention of M. tuberculosis infection57 and the recurrence of active TB disease (both pulmonary and extrapulmonary).58
Another aspect to consider is the design of these studies in relation to their comparative efficacy with BCG, which is used as a control in the trials as it is the only approved vaccine for human use and therefore considered the gold standard. It is worth noting that the studies of MTBVAC have been designed with the goal of demonstrating superior efficacy to BCG, while other vaccines like VPM1002 aim for non-inferiority, meaning the validation of possible alternatives without compromising the protection provided by the reference vaccine. However, given the wide range of BCG formulations, data should be interpreted carefully, avoiding extrapolation of results from one formulation to other existing formulations.9
Additionally, both MTBVAC and GamTBvac express ESAT6-CF10 antigens, which can result in cross reactivity with the IGRA diagnostic test. Consequently, there is a need to develop new diagnostic techniques that reduce the number of false positives after vaccination.26,52
Finally, it is important to note that the administration of live attenuated vaccines, such as MTBVAC, VPM1002, or BCG, poses a risk in individuals infected with HIV. Therefore, trials are being planned to study the safety of theses vaccines in this population.20 A new study whose purpose is to evaluate the safety and immunogenicity of MTBVAC in adolescents and adults living with and without HIV in South Africa (HVTN605A5421) is scheduled to begin in early 2024.59
Research priorities in other MTBVAC usesAfter more than two decades of development, MTBVAC is starting Phase 3 efficacy trial in newborns against TB disease. However, its effects go beyond achieving TB prevention.26 In addition to MTBVAC protection against TB, other uses that take advantage of the non-specific effect of live attenuated vaccines are under investigation in pre-clinical studies. Its potential as a treatment for BCG-refractory non-muscle-invasive bladder cancer has been demonstrated, stimulating a tumour-specific immune response mediated by CD4+ and CD8+ T lymphocytes.60 Similarly, its ability to induce a Th1-type immune response, to the detriment of an IgE-producing Th2 response, has demonstrated MTBVAC's ability to reverse established asthma in mice.61 Furthermore, its capacity to induce “trained immunity”62,63 has been studied, which refers to epigenetic changes in primary myeloid cells, enhancing the immune response against non-bacterial stimuli and providing heterologous protection against Streptococcus pneumoniae62 (Table 3).
Studies of other indications and routes of administration of MTBAVC vaccine.
| Author (year) | Study | Aim | Results |
|---|---|---|---|
| Tarancón R et al. (2020)62 | To study MTBVAC trained immunity and protection against experimental lethal pneumonia compared to BCG in mouse model. | To determine the ability of MTBVAC to induce “trained immunity” and offer protection against a heterologous model of pneumococcal pneumonia in mice. | MTBVAC is able to generate metabolic and immunomodulatory effects and epigenomic modifications through mechanisms similar to BCG, providing protection against heterologous infections unrelated to TB, such as Streptococcus pneumoniae. |
| Dijkman K et al. (2021)64 | Pulmonary MTBVAC vaccination in non-human primates | To assess the impact of using MTBVAC inoculated through the respiratory mucosa in primates to determine their ability to induce adaptative immunity. | Pulmonary vaccination with MTBVAC resulted in a local antigen-specific response that has previously been correlated with protection against TB. Both T lymphocytes and antibodies generated by the vaccine showed an enhanced capacity to respond to M. tuberculosis. |
| Tarancón R et al. (2021)61 | Therapeutic efficacy of pulmonary live tuberculosis vaccines against established asthma by subverting local immune environment in mouse model | To evaluate the therapeutic efficacy of intranasal administration of vaccines based on live attenuated mycobacteria in different models of bronchial hyperresponsiveness. | Both BCG and MTBVAC can reverse bronchial hyperresponsiveness in established asthma, where there is high pre-vaccination eosinophilia, by inducing a potent Th1-type immune response. |
| Vierboom MPM et al. (2021)63 | Comparison standard intradermal vaccination to pulmonary router in non-human primates | To assess the impact of BCG and MTBVAC inoculated through the respiratory mucosa in primates to determine their ability to induce “trained immunity”. | The study showed stronger induction of trained immunity by mucosal BCG or MTBVAC administration compared with standard intradermal route in monocytes present in peripheral blood and bone marrow, with inoculation. |
| Moreo E et al. (2022)60 | Study of therapeutic MTBVAC immunotherapy in a mouse model of orthotopic bladder cancer. | To compare the efficacy of anti-tumour treatment with intravesical BCG versus intravesical MTBVAC. | MTBVAC acts as an antitumour treatment in non-muscle-invasive bladder cancer refractory to BCG treatment, and induce eradication of fully established bladder tumours, largely due to the expression of ESAT6 and CFP10. |
On the other hand, its respiratory administration has been studied as an alternative route to the intradermal inoculation used in most studies. In these cases, MTBVAC was able to induce a local antigen-specific response that extended beyond the site of inoculation and has been previously correlated with protection against TB.64 The respiratory administration of MTBVAC could facilitate universal vaccination in the future (Table 3).
ConclusionsMTBVAC emerged as a response to the need for a better preventive strategy than BCG vaccination, given its variable efficacy. Based on the attenuation of M. tuberculosis, MTBVAC showed an optimal safety profile, as well as an adequate degree of reactogenicity and immunogenicity in preclinical animal studies, which supported its entry into human clinical trials in 2012. After 20 years of development, MTBVAC has started Phase 3 clinical trial to evaluate its efficacy in newborns, with plans for a further trial in adolescents and adults in the future.
While it is not the only candidate to have reached this point, its properties and proven potential over the years make it a very promising candidate.
Conflict of interestC.M. is co-inventor on a patent on Tuberculosis Vaccine held by the University of Zaragoza and Biofabri. Biofabri is an industrial partner of University of Zaragoza and exclusive licensee and industrial and clinical developer of MTBVAC. The authors declare that they have no other known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The construction and development of MTBVAC has been continuously financed by Spanish research projects over the last 25 years and European Union R&D projects. The MTBVAC vaccine project is in ongoing collaboration with Biofabri and the TuBerculosis Vaccine Initiative (TBVI) and the US International AIDS Vaccine Initiative (IAVI). Phase 1b/2a in BCG-vaccinated adolescents and adults with and without prior M. tuberculosis infection is conducted in collaboration with IAVI and financed by funds from the NIH and CDRMP of the United States government. Phase 2 dose-defining clinical trial in newborns is funded by the European Union: The European & Developing Countries Clinical Trials Partnership EDCTP2 “RIA-2016-V-1637” MTBVACN2. EDCTP2 has granted funding for MTBVAC Phase 3 efficacy trial through “RIA2019S-2652” MTBVACN3 Efficacy trial: “MTBVAC in Newborns: Randomised, Double-blind Controlled Phase 3 Trial to evaluate the Efficacy, Safety and Immunogenicity of MTBVAC administered in healthy HIV unexposed uninfected and HIV exposed uninfected newborns in Tuberculosis-Endemic Regions of Sub-Saharan Africa.”