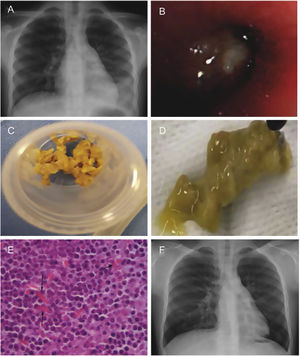
A previously healthy boy was referred when he was 8 years old due to non-resolving left lower lobe (LLL) pneumonia (Fig. 1A), after experiencing five episodes requiring antibiotics in the previous 8 months, showing only temporary clinical improvement.
(A) Chest X-ray in 2017 showing left lower lobe opacity (prior to the first bronchoscopy). (B) Bronchial cast occluding left lower lobe visualized with the flexible bronchoscope. (C and D) Bronchial cast extracted with forceps. (E) Many inflammatory cells, predominantly eosinophils with Charcot-Leyden crystals (arrows). (F) Chest X-ray in 2023 showing small retrocardiac opacity.
A chest computed tomography (CT) exhibited left hilar, subcarinal and bilateral axillary adenopathies with opacification of the LLL. Following a positive interferon-gamma release assay, anti-tuberculosis drugs were started, reaching 6 months of treatment, along with systemic steroids (based on the clinical suspicion of endobronchial tuberculosis).
Three weeks after the CT, a bronchoscopy was performed (July 2017), finding a whitish solid material occluding the LLL bronchus that required forceps and rigid bronchoscopy to be partially removed (Fig. 1B–D). The initial report of the endobronchial plug described it as a compound of fibrinopurulent debris, without evidence of pathogenic microorganisms. Mild blood eosinophilia (1200/μL) was observed.
Concerns arose regarding tuberculosis as the cause of recurrent bronchial casts (BC), so a thorough review of the plugs was done, reporting some fibrino-mucoid material with many inflammatory cells, predominantly eosinophils, along with Charcot-Leyden crystals (Fig. 1E).
Because of reappearing consolidation in the LLL despite treatment with inhaled corticosteroids and azithromycin, serial bronchoscopies were required over 2 years (September 2017, four in 2018, three in 2019), to remove extensive BC. He received oral bursts of steroids (35–60 days/year) from 2017 to 2021.
Owing to the presence of persistent refractory inflammatory BC and blood eosinophilia (500–1300/μL) unrelated to other conditions, treatment with mepolizumab1,2 was deemed suitable. Furthermore, the main components of the casts were eosinophils and bronchoalveolar lavage eosinophilia was present up to 51%.
In May 2020 he needed another bronchoscopy, finding many BC filling all LLL segments. At that point, the patient had only received two doses of mepolizumab, due to poor treatment adherence. After an episode of pneumonia in February 2021, regular monthly treatment with mepolizumab was started. Since then, radiological and clinical improvements were observed.
Some bronchoalveolar lavage and bronchial aspirate cultures were positive for Haemophilus influenzae, but the vast majority were negative. A few colonies of Aspergillus fumigatus were found once in the culture of the BC and bronchial aspirate; however, specific IgE and IgG serum levels were negative.
A chest CT conducted in August 2021 revealed some residual bronchiectasis without significant bronchial occupation. The last bronchoscopy was performed in July 2022, undergone to discard disease progression and settle the therapeutic length with mepolizumab. The only finding was a small and thin cast in a subsegment of S6 in LLL.
In August 2022, a spirometry demonstrates a positive bronchodilator response. A new chest X-ray in March 2023 showed radiological stability, with a small subsegmental retrocardiac opacity and bronchiectasis in LLL, suggesting residual changes (Fig. 1F).
In conclusion, our patient with refractory inflammatory plastic bronchitis3,4 has presented clinical improvement since the beginning of mepolizumab. This treatment with evidenced efficacy in other eosinophilic diseases,1,2 might be helpful in inflammatory plastic bronchitis. Even so, before considering it a standardized treatment, prospective comparative studies are required.
FundingNone declared.
Conflict of InterestsThe authors state that they have no conflict of interests.