Stroma, mainly composed by fibroblasts, extracellular matrix (ECM) and vessels, may play a role in tumorigenesis and cancer progression. Chronic Obstructive Pulmonary Disease (COPD) is an independent risk factor for LC. We hypothesized that markers of fibroblasts, ECM and endothelial cells may differ in tumors of LC patients with/without COPD.
MethodsMarkers of cultured cancer-associated fibroblasts and normal fibroblasts [CAFs and NFs, respectively, vimentin and alpha-smooth muscle actin (SMA) markers, immunofluorescence in cultured lung fibroblasts], ECM, and endothelial cells (type I collagen and CD31 markers, respectively, immunohistochemistry) were identified in lung tumor and non-tumor specimens (thoracotomy for lung tumor resection) from 15 LC-COPD patients and 15 LC-only patients.
ResultsNumbers of CAFs significantly increased, while those of NFs significantly decreased in tumor samples compared to non-tumor specimens of both LC and LC-COPD patients. Endothelial cells (CD31) significantly decreased in tumor samples compared to non-tumor specimens only in LC patients. No significant differences were seen in levels of type I collagen in any samples or study groups.
ConclusionsVascular endothelial marker CD31 expression was reduced in tumors of non-COPD patients, while type I collagen levels did not differ between groups. A rise in CAFs levels was detected in lung tumors of patients irrespective of airway obstruction. Low levels of CD31 may have implications in the overall survival of LC patients, especially in those without underlying airway obstruction. Identification of CD31 role as a prognostic and therapeutic biomarker in lung tumors of patients with underlying respiratory diseases warrants attention.
El estroma, compuesto principalmente por fibroblastos, matriz extracelular (MEC) y vasos, puede desempeñar un papel en la génesis tumoral y la progresión del cáncer. La enfermedad pulmonar obstructiva crónica (EPOC) es un factor de riesgo independiente para el carcinoma de pulmón (CP). Nuestra hipótesis fue que los marcadores de fibroblastos, MEC y células endoteliales pueden variar en los tumores de los pacientes con CP con o sin EPOC.
MétodosSe identificaron los marcadores de fibroblastos asociados al cáncer y los fibroblastos normales cultivados (FAC y FN, respectivamente; marcadores: vimentina y α-actina del músculo liso [SMA por sus siglas en inglés]; inmunofluorescencia en fibroblastos de pulmón cultivados) y marcadores de la MEC y las células endoteliales (marcadores: colágeno tipo I y CD31, respectivamente; inmunohistoquímica) en muestras de pulmón tumoral y no tumoral (toracotomía para resección de tumores pulmonares) de 15 pacientes con EPOC-CP y 15 pacientes con solo CP.
ResultadosEl número de FAC aumentó de forma significativa, mientras que el de FN disminuyó significativamente en las muestras tumorales en comparación con las muestras no tumorales de pacientes con CP y EPOC-CP. Las células endoteliales (CD31) disminuyeron también de forma significativa en las muestras tumorales en comparación con las muestras no tumorales solo en los pacientes con CP. No se observaron diferencias significativas en los niveles de colágeno tipo I en ninguna muestra o grupo de estudio.
ConclusionesLa expresión del marcador vascular endotelial CD31 se redujo en los tumores de los pacientes sin EPOC, mientras que los niveles de colágeno tipo I no difirieron entre los grupos. Se detectó un aumento en los niveles de FAC en los tumores de pulmón de los pacientes, con independencia de la presencia de obstrucción de las vías respiratorias. Los niveles bajos de CD31 pueden tener implicaciones en la supervivencia general de los pacientes con CP, en especial, en aquellos sin obstrucción subyacente de las vías respiratorias. Convendría estudiar e identificar el papel del CD31 como biomarcador terapéutico y de pronóstico en los tumores de pulmón de pacientes con enfermedades respiratorias subyacentes.
Despite recent advances, non-small cell lung cancer (NSCLC) still leads to a great mortality in most of the continents1,2, reaching up to one third of deaths in certain countries1,3. Clinical factors such as chronic obstructive pulmonary disease (COPD) or airway obstruction underlie the pathophysiology of LC in many patients1,4–6. Several relevant investigations have clearly demonstrated that airway obstruction and emphysema render the patients more susceptible to the development of LC7–9. Despite this consolidated knowledge, full elucidation of the underlying biological features is still underway.
In the airways, lungs, and blood compartment of patients with LC and underlying COPD, mechanisms such as redox imbalance, inflammatory events, epigenetics, and immune alterations were shown to be disrupted compared to LC patients with no COPD10. As a result of the interaction of those biological events with key cellular processes, namely angiogenesis, cell death and repair, and the cell survival machinery, COPD patients are more prone to lung tumorigenesis11.
Stroma is defined as the part of a tissue or organ that confers mainly structure with no specific function, and is mainly composed by blood vessels, nerves, and connective tissue. In LC, several components such as extracellular matrix (ECM), endothelial cells, and cancer-associated fibroblasts (CAFs) play a significant role in tumorigenesis and cancer progression12. CAFs are a major component of the stroma in tumors. Growth factors, hormones, and cytokines mediate the tumor cell proliferation favored by CAFs. The most specific and widely used marker of CAFs is alpha-smooth muscle actin (SMA), which is indeed a specific marker of myofibroblasts12. The differentiation process of epithelial cells into mesenchymal cells is known as epithelial-mesenchymal transition (EMT), characterized by the appearance of mesenchymal properties13,14. Interestingly, CAFs may also regulate EMT 13.
Extracellular macromolecules such as collagen, enzymes, and glycoproteins conform a specific network of the ECM, which is also involved in tumor development and progression15. In cancer stroma, collagen was demonstrated to be the most abundant protein16. Importantly, type I collagen promotes growth of cancer cells, invasion, and distant metastasis, thus favoring tumor progression17, as well as resistance to therapy18. Whether a distinct expression of extracellular matrix markers or CAFs may take place in the stroma of lung tumor samples of patients with COPD remains to be answered.
The formation of new vessels in tumors can be identified using specific markers such as platelet endothelial cell adhesion molecule also known as cluster of differentiation (CD) 31. CD31 is involved in several physiological processes, namely maintenance of vascular endothelial and inflammatory cell functions and is also expressed in tumor cells19. In fact, the immunohistochemical measurement of CD31 expression can be reliably used as a marker of neoangiogenesis in tumors20. In mice with experimental airway inflammation mimicking COPD, an immunosuppressive microenvironment of the lung tumors was characterized by increased angiogenesis21. Whether differences in CD31 expression may exist in tumors of patients with COPD compared to non-COPD remain to be identified.
On this basis, we hypothesized that in LC patients with airway obstruction, cancer stroma as analyzed using specific markers may differ from tumors of patients with no underlying COPD. Accordingly, our objectives were to determine in lung tumors and non-tumor specimens (control samples) of LC patients with and without COPD the following parameters: 1) CAFs and non-tumor fibroblasts (cultured fibroblasts), 2) type I collagen as a marker of extracellular matrix, and 3) CD31 expression levels as a marker of endothelial cells and blood vessels.
MethodsStudy design and ethicsThis is a cross-sectional, prospective study designed following the World Medical Association guidelines (Seventh revision of the Declaration of Helsinki, Fortaleza, Brazil, 2013)22 for research on human beings and was approved by the institutional Ethics Committee on Human Investigation (protocol # 2008/3390/I, Hospital del Mar–IMIM, Barcelona, Spain). All patients invited to participate in the study signed the informed written consent. The current investigation followed the international STROBE guidelines23.
Patients were prospectively recruited from the Lung Cancer Clinic of the Respiratory Medicine Department at Hospital del Mar (Barcelona, Spain). All the patients were part of the Lung Cancer Mar Cohort. For this observational study, 30 patients with LC were recruited in 2019. Candidates for tumor resection underwent pulmonary surgery prior to administration of any sort of adjuvant therapy. LC diagnosis and staging were established by histological confirmation and classified according to currently available guidelines for the diagnosis and management of LC24,25. TNM (tumor, node, and metastasis) staging was defined as stated in the 8th edition Lung Cancer Stage Classification26. COPD diagnosis was established as a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ≤ 0.7 which is not fully reversible by spirometry according to currently available guidelines for diagnosis and management of COPD27,28. Exclusion criteria were: small cell lung cancer (SCLC), chronic cardiovascular disease, restrictive lung disease, metabolic, immune disease, or clot system disorders, signs of severe inflammation and/or bronchial infection (bronchoscopy), current or recent invasive mechanical ventilation, or long-term oxygen therapy.
Specimens from the tumor and non-tumor lungs were collected from all the study subjects. Patients were further subdivided post-hoc into two groups on the basis of underlying COPD: 1) 15 patients with LC and COPD (LC-COPD group) and 2) 15 patients with LC without COPD (LC group).
Clinical assessmentIn all patients, lung function parameters were assessed following standard procedures. Diagnosis and severity of patients with COPD were determined according to currently available guidelines6. Nutritional evaluation included the assessment of body mass index (BMI) and nutritional blood parameters from all patients.
Sample collection and preservationLung samples were obtained from tumors and the surrounding non-tumor parenchyma following standard technical procedures during thoracotomy for the standard care in the treatment of lung tumors. In all patients, the expert pulmonary pathologist selected tumor and non-tumor lung specimens of approximately 10x10mm2 area from the fresh samples as previously validated4,29. Non-tumor specimens were collected as far as possible from the lung to the tumor resection margins (average>7cm). Fragments of both tumor and non-tumor specimens were fixed in formalin and embedded in paraffin blocks until further use. Another fragment was harvested in Dulbecco's Modified Eagle Medium (DMEM) with 1% of penicillin, streptomycin, and fungiozone for the cell culture process.
Cell cultureFresh human tumor and non-tumor lung samples were placed in Dulbecco's Modified Eagle Medium (DMEM) with 1% of penicillin, streptomycin, and fungiozone immediately after obtaining lung specimens and transported on ice to the molecular laboratory. Tumor and non-tumor specimens were minced finely and digested in 1% collagenase type I (Sigma-Aldrich, St. Louis, MO) at 37°C for two hours with occasional agitation. Then the digested tissue was centrifuged at 1,200rpm for two minutes. Cell suspensions were cultured on culture plates in proliferation medium consisting of the mixture of DMEM-medium, 10% fetal bovine serum, and 1% penicillin-streptomycin-fungizone solution at 37°C in a 5% CO2 atmosphere. The culture medium was changed after 48hours to remove unattached cells and debris in suspension. Cells were subcultured with 0.025% trypsin (Life Technologies, California, USA) and 0.01% EDTA when they reached 50-80% confluence for ten minutes. All the study experiments were performed on the cultured cells between passages 1 and 2 of the primary cultures to perform immunofluorescence as described below.
Immunoflorescence staining of CAFs and NFsCAFs and NFs were identified by analyzing the fibroblast-specific protein vimentin and alpha-SMA (CAFs). Briefly, cells were fixed with acetone and methanol (1:1) on the slides at -20°C for ten minutes, and were then washed with PBS three times. Subsequently, slides were incubated with blocking solution (50mM Tris with PH=7.5, 150 Mm NaCl, 0.01% Triton, 1% bovine serum albumin and 1% skimmed milk powder) for one hour at room temperature in a humidifed chamber. Subsequently, primary antibodies incubation with anti-alpha SMA antibody (anti-alpha-SMA antibody, Santa Cruz) and anti-vimentin antibody (anti-vimentin antibody, Santa Cruz) was performed overnight at 4°C in the chamber. After washing with PBS three times, slides were incubated with corresponding secondary antibodies diluted in PBS for one hour: anti-mouse IgG FITC (Invitrogen, Thermo Fisher Scientific) and anti-rabbit IgG A647 (Invitrogen, Thermo Fisher Scientific) at room temperature. Finally, the sections were mounted using the fluorescent mounting medium 4’,6-diamidino-2-phenylindole (DAPI) G-Fluoromount medium (Southern Biotech, Birmingham, AL, USA), which specifically stained DNA (allowing identification of all nuclei) in the cell sections. A fluorescence microscope (x 40 objectives, Nikon Eclipse Ni, Nikon, Tokyo, Japan) coupled with a digitizing camera was used to identify and count the number of fibroblasts (30 fields) in each study sample. Results were expressed as the percentage of either both alpha-SMA and vimentin positively stained fibroblasts for identification of CAFs or vimentin-only positively stained for detection of NFs to the total number of counted fibroblasts in the 30 fields. Results are reported separately for both CAFs and NFs in each type of lung specimen and patient group.
Markers of ECM and endothelial cells using immunohistochemistryType I collagen and endothelial cells were identified on three-micrometer lung tumor and non-tumor cross-sections using immunohistochemical procedures as previously described10,29. Following deparaffinization, lung cross-sections were immersed in preheated antigen retrieval solution of ethylenediaminetetraacetic acid (EDTA, pH 9), incubated at 95°C for 40minutes to be then cooled down to room temperature. Slides were washed over the following steps with phosphate buffer saline (PBS). Endogenous peroxidase activity was blocked with 6% hydrogen peroxide for 15minutes. Primary antibody incubation with anti-collagen I antibody (anti-collagen I antibody, Abcam, Cambridge, UK) and anti-CD31antibody (anti-CD31 antibody, Abcam, Cambridge, UK) was performed for one hour. Slides were incubated with biotinylated universal secondary antibody for 30minutes followed by a 30-minute incubation with HRP-streptavidin and diaminobenzidine for five minutes (kit LSAB+HRP Dako Cytomation Inc., Carpinteria, CA, USA) as a substrate. Hematoxylin counterstaining was performed for two minutes and slides were dehydrated and mounted for conventional microscopy. Images of the stained lung sections (tumor and non-tumor) were captured with a light microscope (Olympus, Series BX50F3, Olympus Optical Co., Hamburg, Germany) coupled with an image-digitizing camera (Pixera Studio, version 1.0.4, Pixera Corporation, Los Gatos, CA, USA).
Expression of the markers collagen and CD31 was estimated as the percentage of type I collagen and CD31 using the semiquantitative immunohistochemical scoring system (Hscore) according to methodologies previously published30. Type I collagen and CD31 staining in the tumor and non-tumor specimens was established according to the following categories: Hscore 0 (indicated the absence of staining) and Hscore 1 (indicated the presence of staining). Data are shown as the percentage of both positively and negatively stained structures for all the histological sections in both tumor and non-tumor lung specimens.
Statistical analysesThe normality of the study variables was tested using the Shapiro-Wilk test. The marker CD31 was used to estimate sample size. For the one-way analysis of variance (ANOVA) of one factor, considering the between-group variance to be 10486.7 and the within-group error equal to 3160.9, a minimum of 12 patients (24 patients in total) per type of sample (tumor and non-tumor) sufficed to reach an 80% power given an alpha error of 0.05. The software Stata/MP release 15 (StataCorp LLC, College Station, Texas, USA) was used for sample size calculation. Clinical variables are shown in a Table. Qualitative variables are represented as frequencies (number and percentage), while quantitative variables are shown as mean and standard deviations. Differences in clinical variables between LC and LC-COPD groups of patients were assessed using Student's T-test. Differences among the different biological variables were estimated using ANOVA and Tukey's post-hoc to adjust for multiple comparisons for the two sample types (tumor and non-tumor) and the two patient groups. A subanalysis in which only ex-smokers and non-smokers was conducted. Moreover, one-way covariance (ANCOVA) was also used to adjust for cigarette smoking history in the analyses of all the biological. Statistical significance was established at P ≤ 0.05. All statistical analyses were conducted using the software Statistical Package for the Social Science (SPSS, version 23, SPSS Inc., Chicago, IL, USA).
ResultsClinical characteristicsClinical and functional characteristics of LC and LC-COPD patients are shown in Table 1. Age, sex, or BMI did not significantly differ between the two groups of patients. Ex-smokers and the number of pack-years were significantly greater in LC-COPD patients compared to LC patients, while the number of never smokers was significantly greater in the latter group (Table 1). The lung functional parameters FEV1, FEV1/FVC, DLCO and KCO were significantly lower in LC-COPD than in LC patients (Table 1). Most of the patients were in GOLD stages I and II (93%, Table 1). TNM staging or histological subtypes did not significantly differ between the two groups. In LC-COPD compared to LC patients, the levels of total leukocytes and neutrophils significantly increased, while levels of albumin significantly decreased. Total proteins, fibrinogen, C-reactive protein, globular sedimentation velocity, and body weight loss did not differ between LC-COPD and LC patients.
Clinical and functional characteristics of the study patients.
| Anthropometric variables | LC (N=15) | LC-COPD (N=15) |
|---|---|---|
| Age, years | 67 (10) | 67 (8) |
| Male, N / Female, N | 8 / 7 | 12 / 3 |
| BMI, kg/m2 | 27 (5) | 26 (4) |
| Smoking history | ||
| Current: N, % | 8, 53 | 8, 53 |
| Ex-smoker: N, % | 0, 0 | 7, 47*** |
| Never smoker: N, % | 7, 47 | 0, 0*** |
| Pack-years | 24 (18) | 56 (25)** |
| Lung function parameters | ||
| FEV1, % | 89 (11) | 67 (14)*** |
| FEV1/FVC, % | 76 (5) | 59 (9)*** |
| DLCO, % | 84 (11) | 60 (15)*** |
| KCO, % | 85 (11) | 59 (15)*** |
| GOLD stage | ||
| GOLD Stage I: N, % | NA | 2, 13 |
| GOLD Stage II: N, % | NA | 12, 80 |
| GOLD Stage III: N, % | NA | 1, 7 |
| GOLD Stage IV: N, % | NA | 0, 0 |
| TNM staging | ||
| Stage 0+I: N, % | 8, 53 | 8, 53 |
| Stage II+III: N, % | 7, 47 | 7, 47 |
| Stage IV: N, % | 0, 0 | 0, 0 |
| Histological diagnosis | ||
| Squamous cell carcinoma: N, % | 3, 20.0 | 4, 26.6 |
| Adenocarcinoma: N, % | 10, 66.7 | 10, 66.7 |
| Others: N, % | 2, 13.3 | 1, 6.7 |
| Blood parameters | ||
| Total leucocytes/μL | 6.46 (1.29)×103 | 8.88 (1.84)×103*** |
| Total neutrophils/μL | 4.01 (1.22)×103 | 5.88 (1.74)×103* |
| Total lymphocytes/μL | 1.89 (0.55)×103 | 2.06 (0.81)×103 |
| Albumin (g/dL) | 4.4 (0.22) | 4.0 (0.60)* |
| Total proteins (g/dL) | 6.9 (0.50) | 6.4 (0.74) |
| Fibrinogen (mg/dL) | 441 (160) | 416 (58) |
| CRP (mg/dL) | 3.03 (5.85) | 6.63 (8.61) |
| GSV (mm/h) | 11 (9) | 23 (20) |
| Body weight loss, kg | ||
| 0, N, % | 14, 93.3 | 14, 93.3 |
| 1-5, N, % | 0, 0 | 0, 0 |
| 6-10, N, % | 1, 6.7 | 1, 6.7 |
Continuous variables are presented as mean (standard deviation) while categorical variables are presented as the number of patients in each group and the percentage in the study group total population. Definition of abbreviations: N, number; kg, kilograms; m, metres; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, carbon monoxide transfer; KCO, Krogh transfer factor; GOLD: Global initiative for Chronic Obstructive Lung Disease; NA, not applicable; TNM, tumor, nodes, metastasis; CRP, C-reactive protein; GSV, globular sedimentation velocity; L, liter. Statistical analyses and significance: * p<0.05, *** p<0.001 between LC-COPD patients and LC patients.
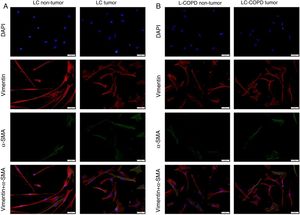
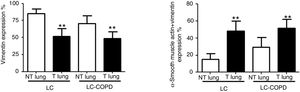
Compared to non-tumor lungs, levels of alpha-SMA significantly increased in tumor specimens both in LC and LC-COPD patients, while levels of vimentin significantly decreased in tumor samples in both groups of patients (Figure 1 and Figure 2).
A and B: Representative examples of immunofluorescence staining of the markers DAPI (upper panel), vimentin (upper middle panel), alpha-SMA (lower middle panel), and CAFs (positively stained for both vimentin and alpha-SMA, bottom panel) in cultured fibroblasts obtained from non-tumor and tumor specimens of LC and LC-COPD patients. Definition of abbreviations: DAPI, 4’, 6-diamidino-2-phenylindole; alpha-SMA, alpha-smooth muscle actin; CAFs, cancer-associated fibroblasts; LC, lung cancer; COPD, chronic obstructive pulmonary disease.
A and B: Mean values and standard deviations (SD) of levels of the markers vimentin and vimentin and alpha-SMA as measured by percentage of the total fibroblasts. Definition of abbreviations: alpha-SMA, alpha-smooth muscle actin; LC, lung cancer; COPD, chronic obstructive pulmonary disease. Statistical analyses: **, p ≤ 0.01 between tumor (T) and non-tumor (NT) lungs in both LC and LC-COPD patients.
Levels of the fibroblast markers alpha-SMA (marker of CAFs) and vimentin (marker of NFs) did not significantly differ in either tumor or non-tumor specimens between LC-COPD and LC patients (Figure 1 and Figure 2).
The subanalysis of the patients according to either GOLD stages or cigarette smoking history revealed identical results to those shown when the entire population was analyzed as a whole (data not shown).
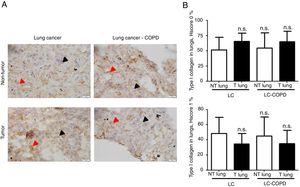
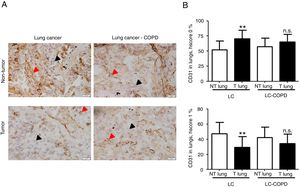
Markers of collagen and endothelial cells in lung specimensLevels of the ECM marker type I collagen and those of the endothelial marker CD31 did not significantly differ in either tumor or non-tumor lungs between the two patient groups (Figures 3 and 4, respectively).
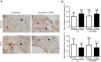
A) Representative examples of immunohistochemical staining for type I collagen in tumor and non-tumor specimens (collagen I-positively stained) in LC and LC-COPD patients, respectively. Black arrows point towards areas stained in blue with hematoxylin (negatively-stained for collagen), while red arrows point towards positively-stained areas (brown color). B) Mean and standard deviations (SD) of levels of type I collagen in tumor and non-tumor of both groups as measured using histoscores (see Methods). Definition of abbreviations: Hscore, histochemical score; LC, lung cancer; COPD, chronic obstructive pulmonary disease. Statistical analyses: n.s., no significance between tumor (T) and non-tumor (NT) lungs in either LC or LC-COPD patients.
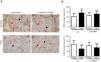
A) Representative examples of immunohistochemical staining for CD31 in tumor and non-tumor specimens (CD31-positively stained) in LC and LC-COPD patients, respectively. Black arrows point towards areas stained in blue with hematoxylin (negatively-stained for CD31), while red arrows point towards positively-stained areas (brown color). B) Mean and standard deviations (SD) of levels of CD31 in tumor and non-tumor of both groups as measured using specific histoscores (see Methods). Definition of abbreviations: Hscore, histochemical score; LC, lung cancer; COPD, chronic obstructive pulmonary disease. Statistical analyses: **, p ≤ 0.01 between tumor (T) and non-tumor (NT) lungs in LC patients; n.s., no significance between tumor (T) and non-tumor (NT) lungs in LC-COPD patients.
Levels of type I collagen did not differ between tumor and non-tumor samples in any study groups of patients (Figure 3). Importantly, in LC patients, levels of Hscore 1 (presence of staining) of CD31 significantly declined in tumor specimens compared to non-tumor samples, whereas those of Hscore 0 (absence of staning) increased (Figure 4). In LC-COPD, no significant differences were seen in CD31 marker leves between tumor and non-tumor samples (Figure 4).
The subanalysis of the patients according to either GOLD stages or cigarette smoking history revealed identical results to those shown when the entire population was analyzed as a whole (data not shown).
DiscussionIn the current investigation, the main findings were that levels of the endothelial marker CD31 significantly decreased in tumors of LC patients, but not in tumors of patients with airway obstruction. In both groups of patients, a rise in the expression of CAFs was seen in lung tumors. Levels of type I collagen in tumor and non-tumor lungs did not differ between patient groups. The most relevant findings collected in the study are discussed below.
CAFs play a crucial role in cancer cell invasion through several mechanisms31. Vimentin, which is expressed in normal mesenchymal cells, maintains cellular integrity and provides resistance against stress. Its function has also been proposed in different cancer cell types including LC32. In the present investigation, the expression of CAFs was significantly greater in the tumor specimens in both groups of LC patients with and without COPD. No significant differences in the levels of cultured CAFs in tumor lungs were seen between the study groups of patients. These findings suggest that CAFs are similarly expressed in lung tumors regardless of underlying airway obstruction. They also imply that fibroblasts are not likely to be involved in an accelerated process of cancer invasion and progression in patients with airway obstruction. Conversely, the percentage of fibroblasts-expressing vimentin-only was significantly reduced in the tumors of both groups of patients. These results also reinforce the concept that CAFs are likely to be a predominant feature of the stroma in lung tumor progression in the patients regardless of the presence of airway obstruction.
Whether a similar profile of CAFs expression can be detected in lung tumors of patients with other underlying respiratory diseases remains to be elucidated. In idiopathic pulmonary fibrosis, myofibroblasts are persistently activated, which secrete collagen type I, and express alpha-SMA fibers, thus they may favor lung tumorigenesis35. Conversely, in patients with non-cystic fibrosis bronchiectasis, a lower or no risk of LC was demonstrated33,34.
Activated myofibroblasts synthesize extracellular components that contribute to the remodeling of the ECM taking place during carcinogenesis. As such CAFs secrete type I collagen, which plays an important role in tumor development, growth, and epithelial-mesenchymal transition36. Moreover, overall survival correlated with low levels of expression of type I collagen and cancer cell differentiation36. In the present study, expression levels of collagen did not significantly differ between tumor and non-tumor samples or between the study groups. These findings suggest that collagen was not a major driver in lung tumor development in these patients, probably because well-differentiated tumor types were analyzed in the study.
CD31 is a glycoprotein expressed in endothelial cells, leukocytes, T cells, and platelets20. CD31 is also expressed in lung tumors37. In the current investigation, a significant decline in CD31 expression levels (Hscore 1) was detected in the tumor specimens of patients with LC, while in patients with underlying airway obstruction no significant differences were seen between lung tumor and non-tumor samples. These findings imply that the vascular endothelial component of stroma was probably involved in the prognosis of LC in patients with and without COPD. In fact, 47% of LC-COPD and 80% of LC patients are still alive in this series (10-year follow-up, data not shown). In keeping with, CD31 has proven to be a useful marker to evaluate angiogenesis in lung tumors38 as well as to monitor the response to specific anti-angiogenic molecules such as vascular endothelial growth factor (VEGF) in clinical settings38,39. In this regard, several investigations have demonstrated that VEGF inhibitors, through reduced angiogenesis (CD31 marker among others), are currently prescribed as single agents in the third-line treatment of patients with NSCLC38,39.
Study limitationsA limitation in the study was the relatively reduced number of analyzed patients. Nonetheless, calculations of sample size estimated 12 patients in each group (24 in total), thus the number of patients included was sufficient to detect statistically significant differences in the study. The degree of airway obstruction might have influenced the study results. However, as most of the patients were in GOLD stages I and particularly II, COPD severity did not exert any significant impact on the results. Almost half of the patients were non-smokers, thus cigarette smoking might have influenced the study results. Nevertheless, a subanalysis in which non-smokers and ex-smokers were included revealed identical results to those obtained with the entire population.
If non-tumor samples had been obtained from a closer distance from the tumors, the profile of biological events might have differed as shown previously for other components of the extracellular matrix (integrins) that probably play a significant role in recurrence40. Nonetheless, this was not explored in the present study, and warrants further attention.
ConclusionsWithin the stroma, the expression of the vascular endothelial marker CD31 was reduced in tumors of patients without airway obstruction, while expression levels of the ECM component type I collagen did not differ between patient groups. A rise in the levels of CAFs was detected in the lung tumors of patients irrespective of underlying airway obstruction.
Low levels of CD31 may have implications in the overall survival of LC patients, especially in those without underlying airway obstruction. Investigations aiming to decipher the specific role of CD31 as a predictor of survival and as a biomarker to monitor anti-angiogenic agents in lung tumors of patients with underlying respiratory diseases are warranted.
Authors’ contributionsStudy conception and design: EB, VC; Patient assessment and recruitment: JT, VC, DRC, MMJ, ARF, RA, LP; Molecular biology analyses: JT, DRC, KA; Statistical analyses and data interpretation: XD, JT, DRC, EB; Manuscript drafting and intellectual input: EB, JT; Manuscript writing final version: EB.
Sources of fundingThis study has been supported by FIS 18/00075 (FEDER, ISC-III) & CIBERES (ISC-III), SEPAR 2018 and SEPAR 2020, and an unrestricted research grant from Menarini SA 2018 (Spain).
Competing interests declared by all the authorsNone.
The authors are thankful to Ms. Mireia Admetlló and Esmeralda Hernández for their help with the patient clinical assessment.