Control status may be a useful tool to assess response to treatment at each clinical visit in COPD. Control status has demonstrated to have long-term predictive value for exacerbations, but there is no information about the short-term predictive value of the lack of control and changes in control status over time.
MethodProspective, international, multicenter study aimed at describing the short-term (6 months) prognostic value of control status in patients with COPD. Patients with COPD were classified as controlled/uncontrolled at baseline and at 3,6-month follow-up visits using previously validated criteria of control. Moderate and severe exacerbation rates were compared between controlled and uncontrolled visits and between patients persistently controlled, uncontrolled and those changing control status over follow-up.
ResultsA total of 267 patients were analyzed: 80 (29.8%) were persistently controlled, 43 (16%) persistently uncontrolled and 144 (53.7%) changed control status during follow-up. Persistently controlled patients were more frequently men, with lower (not increased) body mass index and higher FEV1(%). During the 6 months following an uncontrolled patient visit the odds ratio (OR) for presenting a moderate exacerbation was 3.41 (95% confidence interval (CI) 2.47–4.69) and OR=4.25 (95%CI 2.48–7.27) for hospitalization compared with a controlled patient visit.
ConclusionsEvaluation of control status at each clinical visit provides relevant prognostic information about the risk of exacerbation in the next 6 months. Lack of control is a warning signal that should prompt investigation and action in order to achieve control status.
El estado de control de la enfermedad puede ser una herramienta útil para evaluar la respuesta al tratamiento de la EPOC en cada asistencia a consulta. El estado de control de la enfermedad ha demostrado tener valor predictivo a largo plazo para las exacerbaciones, pero no existe información sobre el valor predictivo a corto plazo de la falta de control de la EPOC y los cambios en dicho control a lo largo del tiempo.
MétodoEstudio prospectivo, internacional, multicéntrico enfocado en describir el valor pronóstico a corto plazo (6 meses) del estado de control de la enfermedad en pacientes con EPOC. Los pacientes con EPOC se clasificaron como con enfermedad controlada/sin controlar al inicio del estudio y en las 3 visitas de seguimiento separadas 6 meses, utilizando criterios de control previamente validados. Se compararon las tasas de exacerbación moderada y grave entre visitas en las que la enfermedad estaba controlada y aquellas en las que no y entre pacientes con control persistente de la enfermedad, pacientes sin control de la enfermedad y aquellos cuyo estado de control cambió durante el seguimiento.
ResultadosSe analizó a un total de 267 pacientes: 80 (29,8%) presentaron control persistente de la enfermedad, 43 (16%) permanecieron con enfermedad no controlada de manera persistente y 144 (53,7%) presentaron un cambio en el estado de control de su EPOC durante el seguimiento. Los pacientes con control persistente de su enfermedad fueron con mayor frecuencia hombres, con un índice de masa corporal más bajo (no elevado) y un FEV1 (%) más alto. Durante los 6 meses posteriores a una visita en la que la enfermedad del paciente no estaba controlada, la odds ratio (OR) para presentar una exacerbación moderada fue de 3,41 (intervalo de confianza [IC] del 95%: 2,47 a 4,69) y la OR=4,25 (IC del 95%: 2,48 a 7,27) para la hospitalización, en comparación con una visita en la que la EPOC estaba controlada.
ConclusionesLa evaluación del estado de control de la EPOC en cada asistencia a consulta proporciona información pronóstica relevante sobre el riesgo de exacerbación en los próximos 6 meses. La falta de control es una señal de alarma que debe motivar la investigación y la acción para lograr el control de la enfermedad.
The most recent update of the Global Strategy for Obstructive Lung Disease (GOLD) recommends reassessing chronic obstructive pulmonary disease (COPD) patients after initial treatment based on symptoms and exacerbations1; however, there is no clear guidance as to how or how often this reassessment should be conducted. The development and validation of a control tool that easily identifies patients who may require a step up/down in treatment and a closer follow-up has been successfully achieved in the management of asthma,2 but the concept of control has been elusive in COPD.3 The main reason for this is the identification of the concept of control with normality in fact, controlled asthma is associated with normal lung function and absence of symptoms.2 While it is obvious that these objectives are not attainable in COPD, it is also true that thanks to smoking cessation, vaccinations and appropriate pharmacological and non-pharmacological treatments many COPD patients may achieve a low level of symptoms and remain stable, without exacerbations and without significant worsening in lung function over prolonged periods of time.4 This status of low level of symptoms (or low impact) and stability is what has been defined as control in COPD.5,6
A recent study demonstrated that the evaluation of control in COPD is more sensitive to clinical changes than changes in GOLD stage, in risk level or in phenotype.7 Moreover, a change in control status over 3 months is followed by significant changes in COPD assessment test (CAT) scores, thereby, making evaluation of COPD control a potential tool to reassess patients at each clinical visit.7
However, the risk of poor outcomes (i.e. exacerbations of different severity) in the short term in association with the uncontrolled status or with the change in status from controlled to uncontrolled has not been previously reported. This is critical to understand the clinical value of the evaluation of control. If uncontrolled status or change to uncontrolled status is associated with increased risk of exacerbations during the next months, this would justify investigating the cause of the lack of control and an intervention to restore the control status and prevent undesirable outcomes.
The current study is the first prospective, international, multicenter study designed with the objective to investigate the concept of control of COPD. In a previous publication we described control status as a marker of increased risk of poor outcomes during follow-up.8 Here, we describe the changes in control status in patients over long-term follow-up, the short-term risk associated with uncontrolled patient visits and the main characteristics of patients that remained either controlled or uncontrolled during the study.
MethodDesign of the studyThis was a prospective international, multicenter study aimed at developing the concept of control in COPD. The design of the study has been described in detail in previous publications.8–10 Eligible patients were recruited to the study and underwent a screening visit (V-1) involving full clinical assessment, including socioeconomic variables, evaluation of current smoking status, current treatment, respiratory symptoms, presence of comorbidities, lung function measured by spirometry and questionnaires. The BODEx (Body mass index (BMI), obstruction, dyspnea and exacerbations) index was calculated.11 A baseline visit (V0) was scheduled after 3 months, in which the control status of the patients was assessed as indicated below. After baseline, the patients were followed with 3 visits at 6-month intervals for a total of 18 months. At each follow-up visit the control status was assessed as at baseline.
At each clinical visit, the CAT12 and the modified Medical Research Council (mMRC) dyspnea scale13 were administered, comorbidities were assessed with the Charlson index14 and physical activity were quantified by self-declared minutes of walking per day.15
A moderate COPD exacerbation was defined as an increase in respiratory symptoms that required the use of systemic corticosteroids and/or a course of antibiotics; when exacerbation required hospital admission it was considered severe.16
The primary outcome of the study was the difference in rates of a composite endpoint during the 18-month follow-up between patients who were controlled versus uncontrolled at baseline. The composite endpoint was defined as occurrence of an ambulatory exacerbation, an emergency room attendance or hospital admission due to an exacerbation, or death. The results of the analysis of the primary outcome have recently been published.8 The current manuscript describes the changes of control status during follow-up and the short-term (6 months) predictive value of the lack of control in patients with COPD.
The study was approved by the local Research and Ethics Committees of each participating center, and all patients provided written informed consent. The data from the United Kingdom (UK) was obtained from the Optimum Patient Care Research Database (OPCRD) and permission to access and link UK data to anonymous electronic medical records was obtained from the Health Research Authority for clinical research use (Anonymised Data Ethics & Protocol Transparency (ADEPT) approval number ADEPT0115). This study was registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP), Register Number EUPAS10679.
PopulationThe inclusion criteria included: age over 40 years, spirometry-defined COPD (i.e. post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC)<0.7), current or ex-smoker of at least 10 pack-years and being in a stable clinical state at the screening visit. Patients were excluded if they: (1) had any chronic concomitant respiratory condition other than asthma or bronchiectasis; (2) were unable to understand the instructions of the study or fill in the questionnaires; (3) had severe comorbidity with a life expectancy shorter than 2 years; or (4) were participating in another clinical study or clinical trial.
Definition of controlA patient was considered controlled when COPD had a low impact and was clinically stable, after adjustment for the severity of the disease.7 Impact was classified as low or high according to the information collected in sputum (presence and color), breathlessness (mMRC), daily physical activity and use of rescue medication. Evaluation of impact was adjusted fordisease severity according to the FEV1. Patients with a FEV1(%)≥50% predicted were classified as mild/moderate and those with FEV1(%)<50% as severe. Stability was defined as the absence of moderate or severe exacerbations in the previous 3 months (Table 1).
Control criteria with adjustment of severity according to FEV1%.
| Control criteria | |
|---|---|
| Low clinical impact(Must meet at least 3 of the 4 criteria) | |
| Dyspnea | 0–1 if FEV1≥50%0–2 if FEV1<50% |
| Rescue medication | ≤3 times/week |
| Sputum color | White or absent |
| Physical activity | ≥30min/day |
| Clinical stability | |
| Moderate or severe exacerbations in the last 3 months. | None |
| Control | Low impact+stability |
Absolute frequencies and percentages were used for comparisons of qualitative variables. The description of quantitative variables was performed using the mean and standard deviation (SD). The Kolmogorov–Smirnov test was used to assess the normality of distributions. In the case of quantitative variables, comparison of the characteristics among groups was carried out using the Student t-test (Mann–Whitney U-test if normality was not assumed). The Chi-squared test (Fisher test for frequencies<5) was employed for the comparison of categorical variables.
Multivariate logistic regression analysis was performed with the independent variable being persistent control status and dependent variables all other demographic and clinical variables, excluding those already used to describe control status.
Odd ratios with 95% confidence intervals (CI) were used to assess the risk of an outcome in the 6 months after an uncontrolled visit compared to a controlled visit. For all the tests p-values<0.05 were considered statistically significant. The statistical package R Studio (V2.5.1) was used for the statistical analyses.
ResultsPopulationA total of 349 patients were screened, 303 (87%) of whom fulfilled all the inclusion and exclusion criteria and could be evaluated for control status at the baseline visit (V0). The mean age was 68.6 years (SD=8.7], 73.9% were male, the mean CAT score was 14.4 (8.6) and the mean FEV1(%) was 52.5% (18.1%). During the 18-month follow-up, 26 patients were lost, and 10 patients died. The characteristics of the whole population and the flow chart of the study have been described in detail in a previous publication.8
Control status at baseline and during follow-upAt baseline, 197 (65%) patients were classified as controlled, 68.5% being mild/moderate and 59.3% severe COPD patients. Of them, 128 (65%) persisted controlled at 6 months, 106 (84% or 54% of the initial 197) at 12 months and 80 (75.4% or 40.6% of the initial 197) at 18 months. From the 106 (35%) uncontrolled at baseline, 70 (66%) persisted uncontrolled at 6 months, 54 (77% or 51% of the initial 106) at 12 months and 43 (79.6% or 40.5% of the initial 106) at 18 months. Therefore, at the end of the 18-month follow-up, 80 (29.8%) patients remained controlled, 43 (16%) persistently uncontrolled and the remaining 144 (53.7%) changed control status during follow-up (Fig. 1).
Characteristics of patients and outcomes according to changes in control status during follow-upIn the univariate analysis, there were significant differences in several variables among patients who were persistently controlled, had variable control status and were persistently uncontrolled (Table 2). Persistently uncontrolled patients were more frequently women, with a higher BMI, more bronchiectasis, worse dyspnea and lung function parameters, more moderate or severe exacerbations in the previous year, few minutes walked per day and worse BODEx and CAT scores.
Demographic and clinical characteristics of patients according to control status at the different follow-up visits.
| Baseline characteristics | All patients(N=267) | Always controlled(N=80) | Variable control(N=144) | Always uncontrolled(N=43) | P value |
|---|---|---|---|---|---|
| Age, years | 68.3 (8.6) | 68.5 (8.7) | 68.3 (8.7) | 68.1 (8.3) | 0.916 |
| Men, n (%) | 196 (73.4) | 72 (90.0) | 102 (70.8) | 22 (51.2) | <0.001 |
| Active smokers, n (%) | 71 (26.6) | 18 (22.5) | 42 (29.2) | 11 (25.6) | 0.549 |
| Pack-years | 47.6 (30.2) | 43.5 (24.3) | 47.8 (31.3) | 54.5 (35.5) | 0.390 |
| BMI (kg/m2) | 26.7 (5.3) | 25.3 (5.2) | 26.9 (5.1) | 28.7 (5.7) | 0.001 |
| Chronic bronchitis, n (%) | 136 (59.9) | 46 (60.5) | 66 (57.9) | 24 (64.9) | 0.829 |
| Emphysema, n (%) | 175 (77.1) | 61 (80.3) | 88 (77.2) | 26 (70.3) | 0.494 |
| ACO, n (%) | 27 (10.1) | 10 (12.5) | 14 (9.7) | 3 (7) | 0.609 |
| Bronchiectasis, n (%) | 35 (13.1) | 15 (18.8) | 10 (6.9) | 10 (23.3) | 0.004 |
| Charlson index | 4.2 (1.6) | 4.0 (1.6) | 4.3 (1.5) | 4.4 (1.8) | 0.261 |
| mMRC | 1.5 (1) | 0.9 (0.8) | 1.1 (0.6) | 1.9 (1) | <0.001 |
| FVC, mL | 2945 (892) | 3303 (865) | 2857 (894) | 2581 (712) | <0.001 |
| FVC (%) | 67.5 (14.5) | 71.6 (15.2) | 66.5 (13.5) | 63.8 (14.1) | <0.001 |
| FEV1, mL | 1512 (582) | 1711 (607) | 1466 (573) | 1302 (459) | <0.001 |
| FEV1 (%) | 53.0 (17.9) | 59.3 (20.1) | 50.5 (16.5) | 49.5 (15.1) | 0.004 |
| Exacerbations in the previous year | 1.4 (2.5) | 0.3 (0.8) | 1.4 (2.2) | 3.6 (3.9) | <0.001 |
| BODEx index | 2.3 (1.7) | 1.5 (1.3) | 2.4 (1.6) | 3.2 (1.8) | <0.001 |
| CAT score | 14.2 (7.9) | 10.8 (5.9) | 13.7 (7.2) | 22.3 (7.9) | <0.001 |
| Minutes walked/day | 88.0 (72.7) | 104.5 (75.3) | 87.5 (74.1) | 58.8 (52.4) | <0.001 |
Footnote: BMI: body mass index; ACO: asthma-COPD overlap; mMRC: modified Medical Research council dyspnea scale; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; BODEx: body mass index, obstruction, dyspnea and exacerbations index; CAT: COPD Assessment Test.
The same trends were observed for outcomes during follow-up in persistently controlled patients not presenting any exacerbation (by definition) and persistently uncontrolled patients presenting a significantly higher number of all types of exacerbations compared with patients with variable control status (Table 3).
Outcomes over the 18-month follow-up of COPD patients according to control status at the different follow-up visits. Exacerbations, emergency visits and hospitalisations are caused by COPD.
| Outcomes during follow-up | All patients(N=271) | Always controlled(N=80) | Variable control(N=144) | Always uncontrolled(N=43) | P value |
|---|---|---|---|---|---|
| Combined event, n (%) | 152 (56.1) | 0 (0) | 109 (75.7) | 40 (93.0) | <0.001 |
| Combined event, mean (SD) | 1.7 (2.6) | 0 (0) | 1.9 (2.3) | 4.2 (3.6) | <0.001 |
| Hospitalization, n (%) | 48 (17.7) | 0 (0) | 28 (19.4) | 19 (44.2) | <0.001 |
| Hospitalization, mean (SD) | 0.3 (0.9) | 0 (0) | 0.3 (0.9) | 0.8 (1.1) | <0.001 |
| Emergency, n (%) | 41 (15.1) | 0 (0) | 26 (18.1) | 14 (32.6) | <0.001 |
| Emergency, mean (SD) | 0.3 (0.8) | 0 (0) | 0.3 (0.8) | 0.7 (1.2) | <0.001 |
| Ambulatory exacerbations, n (%) | 136 (50.2) | 0 (0) | 97 (67.4) | 36 (83.7) | <0.001 |
| Ambulatory exacerbations, mean (SD) | 1.1 (1.6) | 0 (0) | 1.2 (1.3) | 2.7 (2.6) | <0.001 |
Multivariate analysis showed that the characteristics independently and significantly associated with persistent control status were male sex, a lower (not increased) BMI and a better FEV1(%) (Table 4).
Univariate and multivariate logistic regression analyses for controlled patients during follow-up (I).
| Variable | Univariate | Multivariatea | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (+1 year) | 1.00 (0.97–1.03) | 0.836 | – | – |
| Men | 4.57 (2.07–10.08) | <0.001 | 3.20 (1.41–7.28) | 0.005 |
| Pack-years (+1) | 0.99 (0.98–1.00) | 0.150 | – | – |
| BMI (+1kg/m2) | 0.93 (0.88–0.98) | 0.005 | 0.93 (0.88–0.98) | 0.010 |
| Chronic bronchitis | 1.04 (0.59–1.83) | 0.893 | – | – |
| Emphysema | 1.32 (0.67–2.59) | 0.421 | – | – |
| ACO | 1.43 (0.62–3.27) | 0.399 | – | – |
| Bronchiectasis | 1.93 (0.93–3.99) | 0.077 | – | – |
| Charlson index (+1) | 0.87 (0.72–1.05) | 0.141 | – | – |
| FEV1 (+100mL) | 1.09 (1.04–1.14) | <0.001 | 1.07 (1.02–1.12) | 0.006 |
Footnote: OR: odds ratio; CI: confidence interval; BMI: body mass index; ACO: asthma-COPD overlap; FEV1: forced expiratory volume in 1 second.
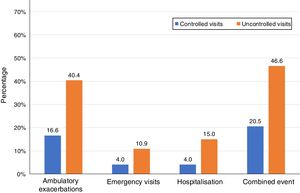
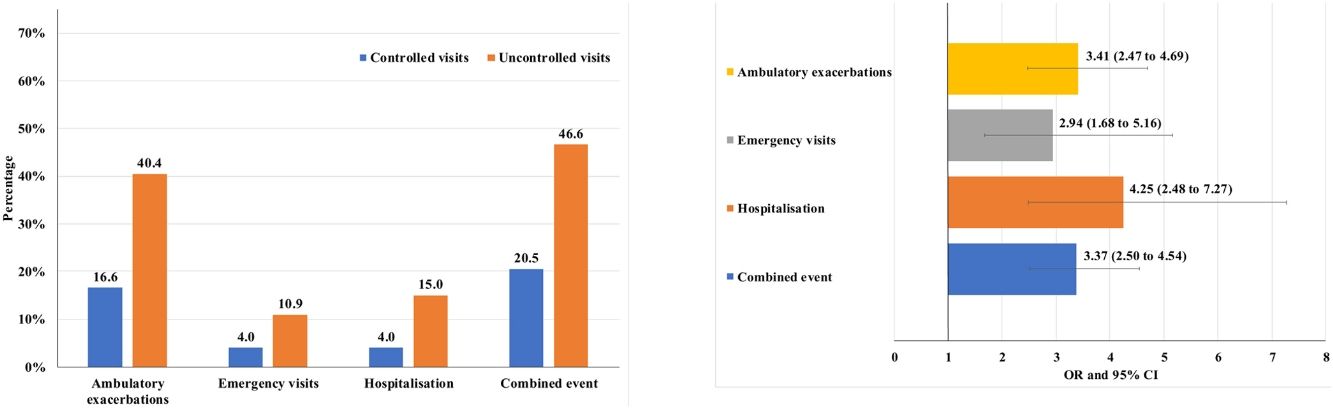
Of the 874 clinical visits with a 6-month follow-up that were valid for analysis of short-term outcomes, 511(58.4%) fulfilled the criteria of controlled and 363 (41.5%) were considered uncontrolled. All the outcomes were more frequent during the 6 months after an uncontrolled patient visit compared to the controlled patient visits (10.9% and 4%, respectively with an emergency room visit and 40.4% and 16.6%, respectively with an ambulatory exacerbation; p<0.001 for both) (Fig. 2). There were 10 deaths, 3 (0.6%) after controlled patient and 7 (1.9%) after uncontrolled patient visits.
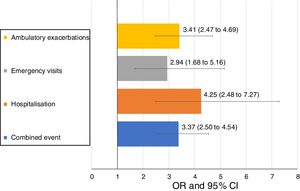
Uncontrolled patient visits resulted in a highly significant increased risk of poor outcomes during the next 6 months with an OR between 2.94 for emergency visits to 4.25 for hospitalizations due to exacerbations compared with controlled patient visits (Table 5, Fig. 3).
Short-term outcomes at 6 months after each clinical visit according to control status at that visit. Exacerbations, emergency visits and hospitalisations are caused by COPD.
| Number of visits with outcome during the next 6 months | All follow-up visits up to V2 (N=874) | OR (95%CI) | P value | |
|---|---|---|---|---|
| Controlled visits(N=511) | Uncontrolled visits(N=363) | |||
| Combined event, n (%) | 105 (20.5) | 169 (46.6) | 3.37 (2.50–4.54) | <0.001 |
| Hospitalization, n (%) | 20 (4) | 51 (15) | 4.25 (2.48–7.27) | <0.001 |
| Emergency visits, n (%) | 20 (4) | 37 (10.9) | 2.94 (1.68–5.16) | <0.001 |
| Ambulatory exacerbations, n (%) | 83 (16.6) | 137 (40.4) | 3.41 (2.47–4.69) | <0.001 |
| Deaths (%) | 3 (0.6) | 7 (1.9) | 3.33 (0.86–12.96) | 0.083 |
Footnote: V2: visit 2; OR: odds ratio; CI: confidence interval. Combined event was defined as occurrence of an ambulatory exacerbation, an emergency room attendance or hospital admission due to an exacerbation, or death. Emergency visits and hospitalizations were due to exacerbations of COPD.
We investigated whether having an exacerbation in the previous 3 months (instability) could offer the same predictive value as the control status. Odds ratios for the different outcomes were also significant but of lower magnitude than those of the control status (data not shown). However, there were 119 visits (32.8% of those classified as uncontrolled) that did not have an exacerbation in the previous 3 months and therefore would have been missed as uncontrolled if only the criteria of previous exacerbation had been used.
DiscussionAccording to the previously defined control criteria, more than half of the population of our study was classified as controlled at baseline; however, the control status changed frequently during the 18-month follow-up. Only 30% of patients remained controlled and 16% persistently uncontrolled throughout the observation period, while the control status changed in 54% of patients during follow-up.
Patients who remained controlled over the course of the study were more frequently men, with a normal (not increased) BMI and with a more preserved FEV1(%). Uncontrolled status had a great predictive value for adverse outcomes with 3-fold higher risk of an emergency room visit for an exacerbation, a 3.4-fold increased risk of an ambulatory exacerbation and a 4-fold increased risk of hospitalization during the next 6 months. These results suggest that lack of control is a useful warning sign that should prompt investigation of the causes and intervention in order to minimize the short-term risk of exacerbations.
Control is a term frequently used to describe a status in which the disease has a minimal impact on the health status of the patient and the prognosis is the best possible. Intuitively, when a chronic disease is uncontrolled it should require more careful evaluation and action taking to improve the symptoms and/or prognosis.17,18 This concept has been very well established in asthma2 but has been elusive in COPD for a variety of reasons, mainly because of a lack of control standard. Since COPD is defined as a chronic, progressive, and non fully reversible airflow limitation, control cannot be defined as the normalization of lung function and disappearance of symptoms and exacerbations. Instead, we need to define a tool that includes some easy to obtain clinical characteristics with meaningful thresholds adjusted for severity, that allow the identification of “uncontrolled” patients and which has prognostic value for relevant outcomes. Control in COPD was initially defined as including two dimensions: impact and stability,5 and the criteria were refined and validated in subsequent prospective studies.6,8,19,20
The criteria selected are easy to obtain at each clinical visit and basically consist in variables that patients should be questioned about, such as the level of dyspnea, sputum production and characteristics, the level of physical activity and the use of rescue medication, in addition to the history of previous moderate or severe exacerbations.21 Using the thresholds derived from previous studies,19 it has been demonstrated that control status is a good predictor of long-term outcomes,8,19,20 and that control evaluation based on clinical data is a better predictor than the definition of control based on CAT scores.19,20 Therefore, we used these clinical criteria in the current analysis.
The last revision of the GOLD strategic document separated, for the first time, the recommendations for initial treatment of COPD and changes in treatment based on a reassessment of the patients in subsequent visits.1 In this context, the control evaluation can be a useful tool to reassess the clinical status of the patients and their future risks. A previous study using the same criteria of control demonstrated that the control tool is more sensitive to changes in the clinical status of the patient than changes in phenotype, the level of risk according to the Spanish COPD guidelines,22 or the GOLD A-D classification frequently used to guide therapy.7 In addition, changes in control status were clinically relevant because they resulted in changes in health status over a period of only 3 months.7 However, the importance of the evaluation of control status at each clinical visit was not demonstrated. In this study, we observed that the control status of the patients frequently changes in subsequent clinical visits separated by 6 months, and more importantly, after an uncontrolled patient visit, the risk of having a moderate or severe exacerbation in the following 6 months is increased by 3–4-fold according to the type of episode. This increased risk justifies the use of control evaluation as a warning sign to foster more careful evaluation of the patients and the adoption of therapeutic measures according to the results of these investigations. In contrast, if the patient fulfills the criteria of control during a clinical visit, we may be quite confident that the risk of an adverse outcome during the next months is low. Therefore, the control status could be used to reduce or extend the time period between routine follow-up visits, with uncontrolled patients probably requiring more frequent appointments compared to controlled patients.
Regarding the limitations of the present study, it is important to consider that control status does not provide any specific diagnosis; a patient may be uncontrolled for a variety of reasons. The uncontrolled status is only an alert to take action but does not tell us what action to take. However, the control tool can be an excellent reminder to the clinician about the questions that COPD patients must be asked at each clinical visit and provide a simple prognostic tool. Only one quarter of the population were women, so extrapolation of the results to women must be made with caution.
The results of the current study are in agreement with those of previous studies demonstrating the predictive value of control status. The results obtained in our study highlight the significantly increased risk of moderate or severe exacerbations during the following 6 months in patients classified as uncontrolled. If these results are confirmed in other cohorts, control criteria should be incorporated into clinical practice as a simple to use tool to help reassess COPD patients at each follow-up visit.
Authors’ contributionsConceptualization: MM, VC, AK, DBP. Data curation: MM, CE, AK. Data acquisition: MM, PS, CKR, RWC, VC, JHYT, TSL, BA, CG, JLG-R, AT, MR-R. Formal analysis: MM, CE, AK. Funding acquisition: MM, DBP. Investigation: MM, PS, CKR, RWC, VC, JHYT, TSL, BA, CG, JLG-R, AT, MR-R, JJS-C, DBP. Project administration: MM, AK, DBP. Writing-original draft: MM. Writing-review and editing: PS, CKR, RWC, VC, JHYT, TSL, BA, CG, JLG-R, AT, MR-R, JJS-C, DBP.
FundingThe study was funded by an unrestricted grant from Novartis AG.
Conflict of interestMM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols. PS has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Grifols, Novartis, Roche and Teva, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Grifols, Novartis and Roche.
CK Rhee received consulting/lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok.
RWC has board membership with GSK, Aerogen, Novartis, and Teva Pharmaceuticals; consultancy agreements with, Aerogen, GlaxoSmithKline, Novartis, Teva Pharmaceuticals, and Vitalograph as well as grants and unrestricted funding for investigator-initiated studies from Vitalograph, Aerogen and GlaxoSmithKline.
TL has received speaker and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca and Novartis.
BA reports grants and personal fees from GSK, grants, personal fees and non-financial support from Novartis AG, personal fees and non-financial support from Boehringer Ingelheim and Chiesi, grants, personal fees and non-financial support from Laboratorios Menarini, personal fees from Gebro, Astra- Zeneca, Laboratorios Rovi and Laboratorios Ferrer, outside the submitted work.
JJSC has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Menarini, Novartis, and Pfizer, and consulting fees from AirLiquide, Boehringer Ingelheim, Chiesi, GSK, AstraZeneca, Ferrer and Novartis.
DBP has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartism, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment.
AT has participated as a member of the local COPD advisory board for Astra Zeneca, GlaxoSmithKline, Bayer, Takeda and Novartis and has received meeting/conference travel grants from Boehringer Ingelheim, Novartis, Astra Zeneca and GlaxoSmithKline.
JGR has received speaker fees from Novartis, GSK, Boehringer-Ingelheim, AstraZeneca, Chiesi, ALK, Teva, Menarini, Rovi, Esteve and Pfizer; and consulting fees from Novartis, GSK, Astra-Zeneca, Teva, Boehringer-Ingelheim, ALK and Esteve.
MRR has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Menarini, Mundipharma, Novartis, Pfizer and Teva, and consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis.
The study was designed and coordinated by the Respiratory Effectiveness Group (REG; www.effectivenessevaluation.org; Cambridge, UK) and delivered by Optimum Patient Care (OPC; www.optimumpatientcare.org).
Spain: Marc Miravitlles, Cristina Esquinas, Miriam Barrecheguren, Alexa Nuñez, Hospital Universitari Vall d’Hebron, Barcelona. Bernardino Alcazar, Hospital de Alta Resolución de Loja. Juan Luis García-Rivero, Karina Hueso, Hospital Comarcal de Laredo, Cantabria. Miguel Roman-Rodríguez, Primary Health-care Center Son Pisà. IB-Salut. Palma de Mallorca. Poland: Pawel Sliwinski, Katarzyna Iwan, Jacek Kolakowski, Institute of Tuberculosis and Lung Diseases, Warsaw. Korea: Chin Kook Rhee, Esther Ahn, St Mary's Hospital. Seoul. Singapore: Jessica Tan, Therese Laperre, Karen Tan Li Leng, Nicole Chia, Ong Thun How, SyifaBinte Shamsuddin, Sherine Lim Shu Gim, Yap Chwee Bee, Soh Rui Ya, Singapore General Hospital. Augustine Tee, Jun Jie Yan, Samuel Hong, William Tan, Changi General Hospital. UK: Victoria Carter, Latife Hardaker, Andrew McLaughlin, Optimum Patient Care, Cambridge. Malta: Caroline Gouder, Mater Dei Hospital. Ireland: Richard W Costello, Royal College of Surgeons. Dublin.











![[CS1]Control status and changes in control status at baseline and follow-up visits. [CS1]Control status and changes in control status at baseline and follow-up visits.](https://static.elsevier.es/multimedia/03002896/0000005700000002/v1_202102021106/S0300289620302040/v1_202102021106/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)