Pulmonary sequestration (PS) represents a rare congenital malformation (0.15–6.45% of all pulmonary malformations) usually supplied by a systemic artery, frequently merging from the aorta or one of its branches.1 Vascularization originating from the coronary circulation is extremely rare with less than 20 cases reported – mostly intralobar sequestrations (presence of independent visceral pleural encasing) supplied either by the right coronary or circumflex artery. Diagnosis can be incidental (e.g. abnormal density on chest radiograph) or in the context of ischemic heart disease due to a coronary steal effect, although arrhythmia has also been described.2–5
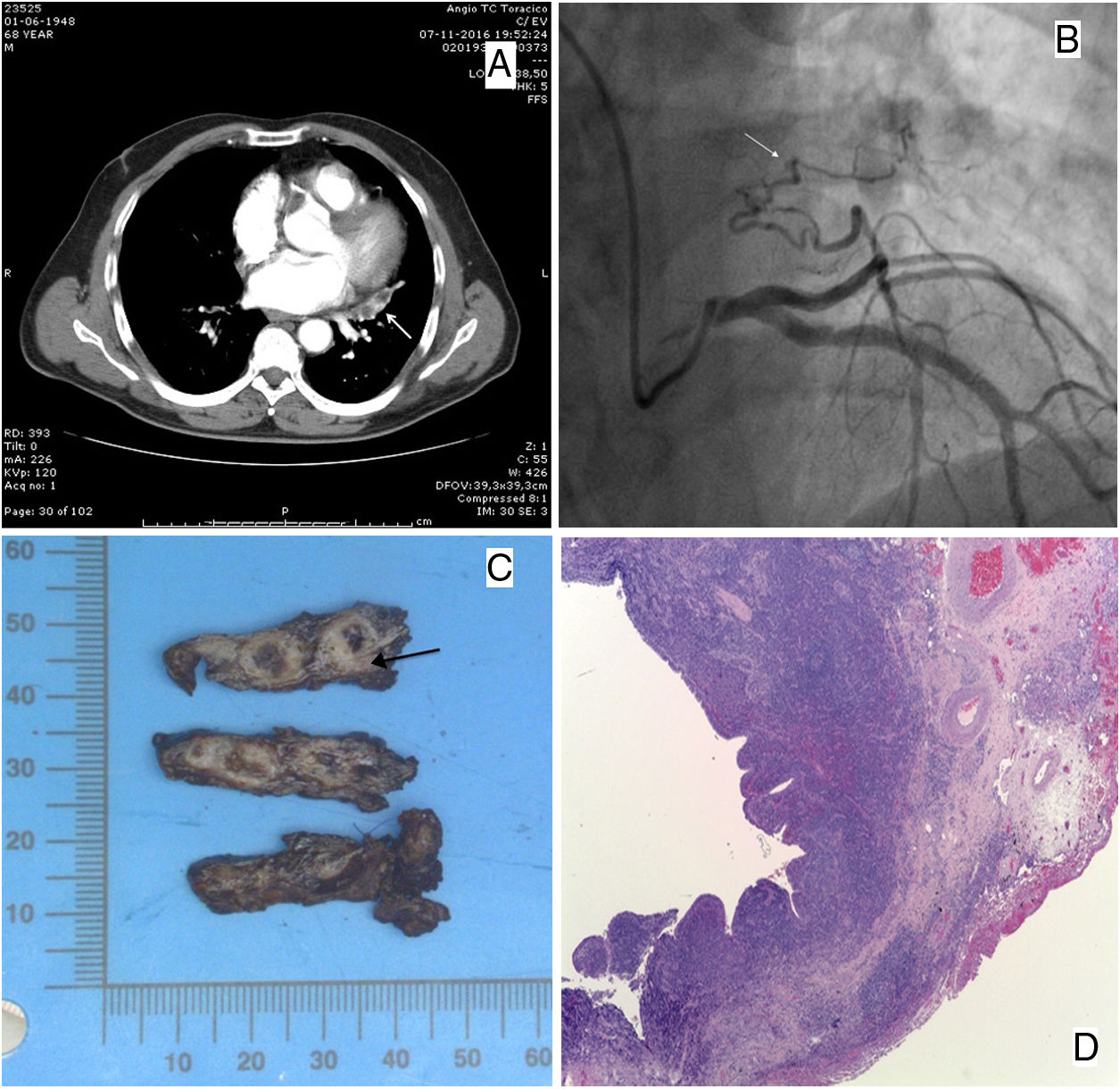
A 68-year-old male with exertion-related chest pain and a recent cardiac stress test suggestive of ischemia (but no confirmation on myocardial scintigraphy), presented in the Emergency Department with a 3-day epigastric pain irradiating to the left hemithorax associated with nausea and dizziness. No remarkable alterations were found on physical examination. The electrocardiogram revealed a sinus rhythm with a slight ST segment depression (<1mm V3–V5); serial measurements of high-sensitivity troponin I were elevated (until T12 with a maximum 2825ng/L). Considering the severe thoracic pain and the difference in blood pressure readings between both arms a thoracic CT-angiography was performed to exclude aortic dissection or pulmonary embolism [despite the stronger possibility of a myocardial infarction (MI)] – a left paracardiac fusiform opacity with non-enhancing areas inside, in the plane of the aortic valve was revealed after contrast administration, with surrounding millimetric vessels in the inferior margin (Fig. 1A); no changes were observed in the adjacent lung parenchyma.
(A) Axial CT in the plane of the aortic valve after contrast revealing a left paracardiac fusiform opacity with non-enhancing areas inside, compatible with bronchial impactions and millimetric vessels surrounding the lesion on the peripheric inferior margin. (B) Coronary catheterization revealing a branch of the left circumflex artery supplying an extracardiac structure in the left lung (arrow). (C) Macroscopy of the surgical resection showing cystic areas surrounded by fibrosis; a systemic artery is present near the base (arrow). (D) (H&E, 25×) Cystic bronchial-like structures, with a respiratory tract epithelial lining, with multifocal erosions and a marked chronic inflammation and fibrosis; intimal and medial hyperplasia in muscular pulmonary arteries.
The patient was hospitalized with the diagnosis of MI (Killip class I) and had no recurrence of symptoms nor rhythm changes on monitorization. Echocardiogram revealed septal and lower wall hypokinesis with preserved left ventricular ejection fraction (62%). Heart catheterization showed an aberrant branch arising from the left circumflex artery (LCA) supplying an extra-cardiac structure on the left lung (Fig. 1B) without other significant hemodynamic stenoses.
The possibility of ischemic heart events due to a steal phenomenon by an anomalous coronary artery arose. A MR angiography (MRA) revealed a 41×20mm mass in the left lower lobe in close contact with the left oblique fissure and with the mediastinum, with arterial vascularization from the LCA, raising the suspicion of an intralobar PS with coronary irrigation. Despite the possibility of occluding the supplying artery through a transcatheter procedure, considering the risk of infection/necrosis (and eventually malignancy), the patient underwent left lateral thoracotomy – an intralobar PS with supply from an aberrant branch from the left circumflex artery was identified and the lesion was excised. Macroscopic examination showed cystic areas surrounded by fibrosis (Fig. 1C). Histology revealed cystic bronchial-like structures surrounded by respiratory epithelium with fibrosis and chronic inflammation (Fig. 1D). Myocardial scintigraphy after surgery showed no signs of myocardial ischemia. The patient remained asymptomatic ever since.
A PS supplied by a coronary artery (PSsCA) can theoretically cause symptoms of ischemic heart disease (IHD) through a mechanism of blood steal (even in the absence of significant stenotic coronary vessels) as reported by Nakayama et al.6 In the few cases published, most patients presented with symptoms of IHD on exertion or even while resting, which prompted a cardiac catheterization. In two cases manifestations included frequent episodes of ventricular tachycardia and of bradycardia (due to sick sinus syndrome) – the former treated with radio-frequency ablation and angioplasty, the latter received a pacemaker.4,5 A history of recurrent respiratory infections is not uncommon, usually beginning at a very young age (more often with intralobar sequestrations).7–9 Hemoptysis as a PS manifestation has been reported (with massive hemoptysis being uncommon but a potentially serious event) and can result from structural changes (like bronchiectasis) or even pulmonary hypertension.3,10
Chest CT provides the best display of the airways and parenchymal abnormalities in PS – they most commonly appear as a homogeneous or inhomogeneous mass, with or without cystic changes and less frequently as multiple small cystic lesions or a large cavitary lesions with air-fluid level.11 Identification of the aberrant artery is crucial, either for diagnosis (as PS can mimick a malignant tumor) and for preoperative assessment considering the risk of accidental incision and hemorrhage; lack of visualization may happen with smaller size vessels (<1mm) or with an unfavorable orientation.11,12 Multidetector CT angiography usually reveals both the arterial supply and the venous drainage, making this a diagnostic procedure of choice.11–13 MRI also has the hability to demonstrate the precise anatomic localization as well as the arterial and venous course. However cystic or emphysematous changes close to the sequestration may not be well delineated and respiratory artifacts can cause low spatial resolution – breath-hold contrast-enhanced MRA can overcome the last one and be as adequate as a CT angiography for vascular characterization.13 Chest radiography has not the diagnostic value of the previous image techniques but abnormal findings can motivate further investigation.11 In the case described (as in most cases with PSsCA) the patient presented with signs and symptoms suggestive of ischemic heart disease and the abnormal irrigation was firstly revealed during heart catheterization. The MRA, together with the CT and the cardiac catheterization findings, supported the possibility of an artery originating from the LCA area.
Imaging differential diagnosis generally includes lung cancer, pulmonary cysts or mediastinal tumors. Regarding PSsCA in particular coronary-bronchial artery fistulas (CBF) are another differential diagnosis to consider. These are congenital anastomoses usually found incidentally during invasive coronary angiography and are often associated with bronchiectasis.14 Most CBF are clinically silent but can become hemodynamically significant in association with a variety of cardiovascular diseases such as cardiomyopathies or supravalvular aortic stenosis. Chest pain and dyspnea related to steal-phenomenon and hemoptysis are the most common symptoms.14 Diagnosis can be achieved using the same image modalities as for PSsCA.14
Surgical resection is recommended in symptomatic patients although coil embolization of the feeder artery (during angioplasty) is also an option. In the case presented, surgery was preferred considering the symptoms, the risk of infection and also of cancer – a few cases of malignant neoplasms being involved in or near sequestered segments have been reported.15